RELISTOR 12 mg/0.6 ml INJECTABLE SOLUTION

How to use RELISTOR 12 mg/0.6 ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Relistor 12 mg/0.6 ml Solution for Injection
Methyl Naltrexone Bromide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.,keep this leaflet. You may need to read it again.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Relistor and what is it used for
- What you need to know before you use Relistor
- How to use Relistor
- Possible side effects
- Storage of Relistor
- Contents of the pack and other information
1. What is Relistor and what is it used for
Relistor contains the active substance methyl naltrexone bromide, which works by blocking the unwanted effects of opioid pain medicines on the gut.
It is used to treat constipation caused by certain types of strong painkillers, such as morphine or codeine, in adults. It is used when other laxative medicines have not worked. Your doctor will have prescribed you opioids. Your doctor will tell you whether you should continue with your usual laxative therapy or stop it when you start taking this medicine.
This medicine should only be used in adults (18 years of age and older).
2. What you need to know before you use Relistor
Do not use Relistor
- if you are allergic to methyl naltrexone bromide or any of the other ingredients of this medicine (listed in section 6).
- if you or your doctor suspect that you have or have had a bowel obstruction or that your abdomen is in a condition that requires immediate surgical intervention (this condition must be diagnosed by your doctor)
Warnings and precautions
Talk to your doctor or pharmacist before using Relistor
- If you have severe stomach symptoms that persist or worsen, contact your doctor immediately as it could be a sign of a hole in the wall of the intestine (intestinal perforation). See section 4.
- If you have Crohn's disease or a gastrointestinal ulcer.
- If you feel unwell, vomit, tremble, sweat, have abdominal pain and/or a fast heartbeat soon after taking Relistor, talk to your doctor.
- If you have severe liver or kidney disease.
- If you start to have severe or persistent diarrhea (repeated watery stools), stop treatment and consult your doctor immediately.
- It is important that you have a bathroom nearby and, if needed, have help available, as you may have a bowel movement within 30 minutes after injection of the medicine.
- Tell your doctor if you have persistent stomach pain, nausea, or vomiting, whether they are new or worsening.
- Tell your doctor if you have had a colostomy, have a peritoneal catheter, or suffer from diverticular disease or fecal impaction, as this medicine should be used with caution in these cases.
- If you are receiving palliative care for advanced disease, this medicine should only be used for a limited time, usually less than 4 months.
- This medicine should not be used to treat constipation that is not caused by opioid use. Tell your doctor if you already had constipation before taking opioid pain medicines.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age, as the risks and benefits are unknown
Using Relistor with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Your doctor may allow you to take other medicines, including those you were using before for constipation.
Pregnancy and breastfeeding
The effects of methyl naltrexone bromide on pregnant women are not known.
Your doctor will decide whether you can use Relistor if you are pregnant.
Women using this medicine should not breastfeed, as it is not known whether methyl naltrexone bromide passes into breast milk.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Dizziness is a common side effect of this medicine. This may affect your ability to drive or use machines.
Important information about some of the ingredients of Relistor
This medicine contains less than 23 mg (1 mmol) of sodium per dose, which is essentially "sodium-free".
3. How to use Relistor
Follow exactly the administration instructions for this medicine given by your doctor.
If you are unsure, consult your doctor or pharmacist again.
The recommended dose for patients with chronic pain (except patients receiving palliative care for advanced disease) is 12 mg of methyl naltrexone bromide (0.6 ml of solution) administered by subcutaneous injection (under the skin), as needed, at least 4 times a week and no more than once a day (7 times a week).
The recommended dose for patients receiving palliative care for advanced disease is 8 mg of methyl naltrexone bromide (0.4 ml of solution) for patients weighing between 38-61 kg or a dose of 12 mg (0.6 ml of solution) for patients weighing between 62-114 kg. The dose is administered every 48 hours (every 2 days) by subcutaneous injection (under the skin).
Your doctor will determine the dose.
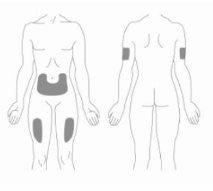
This medicine is administered by injection under the skin (subcutaneous injection) in: (1) the top of the legs (thighs), (2) the abdomen (below the navel), and (3) the top of the arms (if you do not inject yourself). (See INSTRUCTIONS FOR PREPARING AND ADMINISTERING A RELISTOR INJECTION).
After you receive the injection, you may have a bowel movement within a time frame that varies from a few minutes to several hours. Therefore, it is recommended that you have a bathroom nearby.
If you use more Relistor than you should
If you have used more medicine than you should (either because you have injected too much at one time or used more than one injection in 24 hours), you may feel dizzy when standing up, so inform a doctor or pharmacist immediately. Always carry the packaging of the medicine with you, even if it is empty.
If you forget to use Relistor
If you forget to administer a dose, inform your doctor or pharmacist as soon as possible. Do not take a double dose to make up for forgotten doses.
If you stop using Relistor
Consult a doctor or pharmacist if you want to stop treatment with this medicine.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
There have been reports of holes in the wall of the intestine (gastroduodenal perforation) in patients using Relistor. The frequency of this event is unknown from the available data. If you experience severe stomach pain, stop taking this medicine and call your doctor immediately.
The following side effects are very common and may affect more than 1 in 10 people. If you experience any of these side effects intensely or persistently, consult your doctor:
- Abdominal pain (stomach pain)
- Nausea
- Diarrhea (frequent watery stools)
- Flatulence (gas, bloating)
Other common side effects that may affect up to 1 in 10 people are:
- Dizziness (lightheadedness)
- Symptoms similar to opioid withdrawal (such as feeling cold, chills, runny nose, sweating, goosebumps, flushing, rapid heartbeat)
- Injection site reactions (e.g., itching, burning, pain, redness, swelling at the site where the medicine was injected)
- Vomiting
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Relistor
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and vial after EXP. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
Keep the vial in the outer carton to protect it from light.
Only use this medicine if the solution is clear, colorless to pale yellow, and does not contain particles or sediment.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Relistor
- The active ingredient is methylnaltrexone bromide. Each 0.6 ml vial contains 12 mg of methylnaltrexone bromide. One ml of solution contains 20 mg of methylnaltrexone bromide.
- The other components are sodium chloride, sodium edetate and calcium, glycine hydrochloride, water for injection, hydrochloric acid (for pH adjustment) and sodium hydroxide (for pH adjustment).
Appearance of the Product and Container Contents
Relistor is an injectable solution. It is transparent, colorless to light yellow and does not contain particles or sediment.
Each vial contains 0.6 ml of solution.
Packages containing more than one vial have inner cardboard packages with: one vial, a 1 ml syringe with retractable needle and two alcohol-impregnated swabs.
The following presentations are available:
1 vial
Package with 2 vials, 2 injection syringes with retractable needles and 4 alcohol-impregnated swabs (contains 2 inner cardboard packages)
Package with 7 vials, 7 injection syringes with retractable needles and 14 alcohol-impregnated swabs (contains 7 inner cardboard packages).
Only some package sizes may be marketed.
Marketing Authorization Holder
Bausch Health Ireland Limited
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Manufacturer
Bausch Health Ireland Limited
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Bausch Health Poland Sp. z o. o.,
ul. Przemyslowa 2,
35-959 Rzeszów,
Poland
Przedsiebiorstwo Farmaceutyczne Jelfa SA
ul. Wincentego Pola 21
58-500 Jelenia Góra,
Poland
Date of the Last Review of this Leaflet: 11/2023
Detailed information on this medicinal product is available on the European Medicines Agency (EMA) website http://www.ema.europa.eu/.
QUESTIONS FOR THE PATIENT
This section contains important questions that you will have to answer before using and during treatment with Relistor.
If you answer "No" to any of the following questions during the course of your treatment with the medicine, please contact your doctor, nurse or pharmacist.
- Are you receiving opioid therapy (such as morphine or codeine) for your illness?
- Has it been 48 hours or more since your last bowel movement?
- Are you familiar with the self-injection technique or has your doctor (or nurse or pharmacist) explained it to you?
- Do you have sufficient mobility to reach the bathroom, or do you have someone to care for you and help you?
- Do you have the contact number of your health center?
INSTRUCTIONS FOR PREPARING AND ADMINISTERING A RELISTOR INJECTION
This section is divided into the following subsections:
Introduction
Stage 1: Pre-injection Instructions
Stage 2: Syringe Preparation
Stage 3: Selection and Preparation of the Injection Site
Stage 4a: Injection of Relistor using the Package with Syringe and Retractable Needle
Stage 4b: Injection of Relistor using a Normal Syringe and Needle
Stage 5: Disposal of Waste
Introduction
The following instructions explain how to inject Relistor. Please read them carefully and follow them step by step. Your doctor, nurse or pharmacist will instruct you on the self-administration techniques. Do not attempt to administer an injection until you are sure you know how to do it. This injection should not be mixed in the same syringe with any other medicine.
You may have a package that contains an inner cardboard package with everything you need to inject or, on the other hand, you may only have the vial of medicine. If you only receive the vial, you will need several cotton balls (swabs) with alcohol and an injection syringe.
Stage 1: Pre-injection Instructions
- Choose a flat, clean and well-lit surface where you can place the contents of your Relistor package. Make sure you have enough time to complete the injection.
- Wash your hands thoroughly with soap and lukewarm water.
|
- Prepare the necessary materials for the injection, the Relistor vial, a 1 ml syringe for subcutaneous injection (with or without retractable needle), 2 alcohol-impregnated swabs and a gauze or cotton ball.
- Make sure the solution inside the vial is transparent, colorless to light yellow and does not contain visible particles. If it is not, do not use the solution. Contact your pharmacist, nurse or doctor for advice.
Stage 2: Syringe Preparation
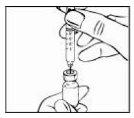
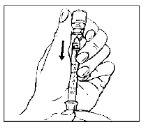
- Remove the plastic protective cap from the vial.
|
- Clean the rubber stopper of the vial with an alcohol-impregnated swab and place it on the work surface. Make sure not to touch the rubber stopper again.
- Take the syringe from your work surface. Hold the cylinder (body) of the syringe with one hand and pull the needle cap straight out. Leave the needle cap on the work surface. DO NOT touch the needle or let it come into contact with any other surface.
|
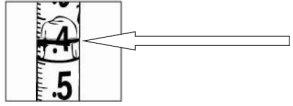
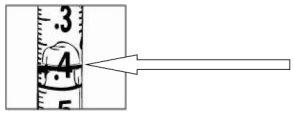
Carefully pull the plunger of the syringe all the way to the 0.4 ml mark for 8 mg of Relistor or to the 0.6 ml mark for 12 mg of Relistor. Your doctor, nurse or pharmacist will have informed you of the dose that has been prescribed for you and how often you should inject it. For patients receiving palliative treatment for advanced disease, the most common doses are included in the following table. The dose is usually given every 48 hours (every two days) by subcutaneous injection (under the skin).
Patient weight in kg | Fill the syringe to the ml level (dose) |
Less than 38 kg | 0.15 mg/kg |
38-61 kg | 0.4 ml (8 mg) |
62-114 kg | 0.6 ml (12 mg) |
More than 114 kg | 0.15 mg/kg |
For patients with chronic pain (except patients receiving palliative treatment for advanced disease), load the syringe to the 0.6 ml mark for 12 mg of Relistor.
| Carefully pull the plunger to the correct mark on the syringe (e.g. 0.4 ml if you have been prescribed 8 mg) |
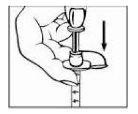
- Insert the needle straight down into the center of the vial stopper. Do not insert the needle at an angle, as it may bend or break. To prevent it from slipping, hold the vial on the work surface with your other hand. You will feel a slight resistance when the needle passes through the stopper. Check that the tip of the needle is inside the vial.
|
- To expel air from the syringe, gently push the plunger down to inject air into the Relistor vial.
|
- If you are using the syringe with a retractable needle provided, DO NOT PUSH THE PLOUGH TO THE BOTTOM. Stop pushing the plunger when you feel resistance. If you push the plunger to the bottom, you will hear a "click", which means that the safety mechanism has been activated, after which the needle will disappear into the syringe. If this happens, discard the product and start again with another vial and another syringe.
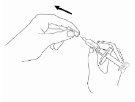
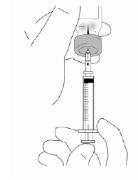
With the needle still in the vial, turn the vial completely over (see figure). Keep the syringe at eye level so that you can see the dosage marks and make sure the tip of the needle is always inside the liquid. Carefully pull the plunger to the 0.4 ml or 0.6 ml mark on the syringe, or as advised, depending on the dose prescribed by your doctor, nurse or pharmacist. You may notice that a little liquid or bubbles remain inside the vial once the syringe is properly filled, this is normal.
|
- With the needle still in the Relistor vial, check if there are air bubbles in the syringe. If there are bubbles, gently tap the syringe with a finger to make them rise to the top; continue holding the Relistor vial and syringe. Gently push the plunger up until all the air bubbles come out. If you spill some of the solution into the vial, carefully pull the plunger back to extract the correct amount of solution back into the syringe. Due to the safety design of the syringe, a small air bubble may resist extraction. Do not worry about this, as it will not affect the accuracy of the dose or pose a risk to your health.
| Tap the syringe upside down and eliminate all air bubbles by pushing the plunger up |
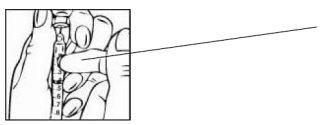
- Always make sure you have the correct dose in the syringe. If you are not sure, contact your doctor, nurse or pharmacist.
| Make sure you have the correct dose in the syringe (e.g. 0.4 ml if you have been prescribed 8 mg). |
- Remove the syringe and needle from the vial. Keep the needle attached to the syringe. Do not touch the needle or let it come into contact with any surface. Once the medicine is drawn into the syringe, it must be used within 24 hours, as Relistor is affected by light and may not work properly if left in the syringe for more than 24 hours.
|
Stage 3: Selection and Preparation of the Injection Site
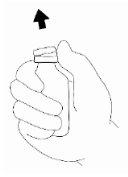
- The three areas of the body recommended for Relistor injection are: (1) upper thighs (thighs), (2) abdomen (belly) and (3) upper arms (only if someone else is giving you the injection).
|
- It is recommended to change the injection site each time an injection is given. Avoid repeated injections exactly at the same site used previously. Do not inject into areas where the skin is sensitive, bruised, red or hard. Avoid areas with scars or stretch marks.
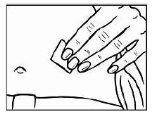
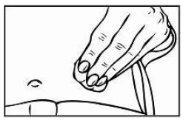
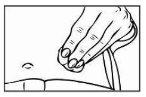
- To prepare the skin area where you will inject Relistor, clean the injection site with an alcohol-impregnated swab. DO NOT TOUCH THIS AREA AGAIN BEFORE ADMINISTERING THE INJECTION. Let the injection site air dry before injection.
|
Stage 4a: Injection of Relistor using the Package with Syringe and Retractable Needle
- Hold the filled syringe with the needle pointing upwards, and re-examine the syringe to see if it contains air bubbles. If there are bubbles, gently tap the syringe with a finger until the air bubbles rise to the top of the syringe. Slowly push the plunger up to expel the air bubbles from the syringe.
- Hold the syringe with one hand as if it were a pencil. With the other hand, gently pinch a clean area of skin and hold it firmly.
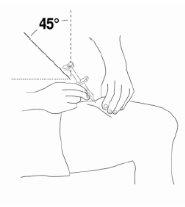
- Insert the entire needle into the skin at a slight angle (45 degrees) with a quick and short movement.
|
- Once the needle is inserted, release the skin pinch and slowly push the plunger all the way down until the syringe is empty and you hear a click.
- When you hear a "click", it means that all the contents have been injected, after which the needle automatically retracts from the skin and is covered (inside the syringe). A small bleeding may occur at the injection site. Press a gauze or cotton ball over the injection site. Do not rub the injection site. If necessary, you can cover the injection site with a bandage.
|
Stage 4b: Injection of Relistor using a Normal Syringe and Needle
- Hold the filled syringe with the needle pointing upwards, and re-examine the syringe to see if it contains air bubbles. If there are bubbles, gently tap the syringe with a finger until
the air bubbles rise to the top of the syringe. Slowly push the plunger up to expel the air bubbles from the syringe.
- Hold the syringe with one hand as if it were a pencil. With the other hand, gently pinch a clean area of skin and hold it firmly.
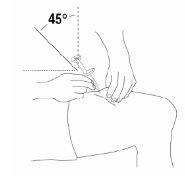
- Insert the entire needle into the skin at a slight angle (45 degrees) with a quick and short movement.
|
- Once the needle is inserted, release the skin pinch and slowly push the plunger all the way down to inject Relistor.
- Once the syringe is empty, quickly remove the needle from the skin at the same angle as it was inserted. A small bleeding may occur at the injection site. Press a gauze or cotton ball over the injection site. Do not rub the injection site. If necessary, you can cover the injection site with a bandage.
|
Stage 5: Disposal of Waste
The syringe with the retracted needle or the syringe and needle MUST NOT be reused. NEVER put the needle cap back on. Dispose of the syringe with the retracted needle or the needle and syringe in a puncture-resistant container as directed by your doctor, nurse or pharmacist.
- Country of registration
- Average pharmacy price37.12 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RELISTOR 12 mg/0.6 ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 12 mgActive substance: methylnaltrexone bromideManufacturer: Bausch Health Ireland LimitedPrescription requiredDosage form: TABLET, 12.5 mgActive substance: naloxegolManufacturer: Grünenthal GmbhPrescription requiredDosage form: TABLET, 25 mgActive substance: naloxegolManufacturer: Grünenthal GmbhPrescription required
Online doctors for RELISTOR 12 mg/0.6 ml INJECTABLE SOLUTION
Discuss questions about RELISTOR 12 mg/0.6 ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions