
REACTINE LEVOCABASTINE 0.5mg/ml EYE DROPS, SUSPENSION

How to use REACTINE LEVOCABASTINE 0.5mg/ml EYE DROPS, SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
REACTINE Levocabastina 0.5 mg/ml Eye drops, suspension
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Follow exactly the administration instructions of the medicine contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or more information, ask your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet (see section 4).
You should consult your doctor if it worsens or does not improve after 2 days.
Contents of the package leaflet:
- What is REACTINE Levocabastina and what is it used for
- What you need to know before you start using REACTINE Levocabastina
- How to use REACTINE Levocabastina
- Possible side effects
- Storage of REACTINE Levocabastina
- Contents of the pack and further information.
1. What is REACTINE Levocabastina and what is it used for
REACTINE Levocabastina belongs to a group of medicines called antiallergic agents.
It is indicated for the temporary relief of symptoms of allergic conjunctivitis such as: tearing, itching, and redness of the eyes.
You should consult a doctor if it worsens or does not improve after 2 days.
2. What you need to know before you start using REACTINE Levocabastina
Do not useREACTINE Levocabastina
If you are allergic to levocabastina or to any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use REACTINE if you have any kidney disease, as the medicine is mainly eliminated through the kidneys.
As with any ophthalmic preparation containing Benzalkonium chloride, propylene glycol, and esters, while being treated with REACTINE Levocabastina, do not use soft (hydrophilic) contact lenses.
Interference with laboratory tests:
If you are going to have any diagnostic test (including blood tests, urine tests, skin tests using allergens, etc.), inform your doctor that you are using this medicine, as it may alter the results.
Using REACTINE with other medicines
Tell your doctor or pharmacist if you are taking, have taken, or may take any other medicine.
No interactions of REACTINE Levocabastina with other medicines have been reported, but in any case, it should be separated by at least 10 minutes from the administration of any other ophthalmic preparation.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medicine.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
You may notice in your eyes: irritation, pain, inflammation, itching, redness, burning sensation, and blurred vision immediately after applying REACTINE. Do not drive or use machines until these effects have disappeared.
REACTINE Levocabastinacontains benzalkonium chloride
This medicine may cause eye irritation because it contains benzalkonium chloride, propylene glycol, and esters.
Avoid contact with soft contact lenses.
Remove contact lenses before application and wait at least 15 minutes before putting them back.
It alters the color of soft contact lenses.
3. How to use REACTINE Levocabastina
Follow exactly the administration instructions of the medicine contained in this leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
Adults and adolescents over 12 years: 1 drop in each eye 2 times a day (every 12 hours). If relief of symptoms is not maintained for 12 hours, the number of applications can be increased to 3 or 4 times a day (every 6 or 8 hours) as needed.
Do not exceed 4 drops in each eye per day (in 24 hours).
Use in children:
This medicine should not be used in children under 12 years.
Method of use:
This medicine is used by the ophthalmic route (in the eyes).
Shake the bottle before use.
With clean hands, open the container, making sure the cap and dropper do not touch anything.
With your head tilted back, separate the lower eyelid, press the bottle carefully until you see 1 drop fall into the eye while looking up. Try to blink to spread the drop throughout the eye.
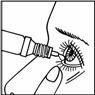
The application of the eye drops should be done with the utmost cleanliness, avoiding any contact with the dropper (e.g., eyelids, fingers, etc.)
After each application, close the container well.
Since this is a sterile medicine, it is recommended to follow these instructions:
- each container should only be used by one patient
-once the treatment is finished, discard the medicine even if the contents of the bottle have not been completely used.
If you use moreREACTINE Levocabastinathan you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist, go to a medical center, or call the Toxicology Information Service (telephone: 91-562 04 20), indicating the medicine and the amount ingested.
If you forget to use REACTINE Levocabastina
If you forget to use REACTINE Levocabastina, do not use a double dose to make up for the forgotten dose. If necessary, use the medicine again as indicated in section 3. HOW TO USE REACTINE Levocabastina
4. Possible side effects
Like all medicines, REACTINE Levocabastina can cause side effects, although not everybody gets them.
- Frequent may affect up to 1 in 10 patients: eye pain, blurred vision
- Uncommon may affect up to 1 in 100 patients: eyelid swelling
- Very rare may affect up to 1 in 10,000 patients: palpitations, hypersensitivity, headache and eye pain, conjunctivitis, watery eyes, allergic reaction, angioedema (swelling of certain areas of the skin), contact dermatitis, urticaria, and reactions at the application site such as burning sensation of the eye, redness, irritation, inflammation, itching, and eye pain, and blurred vision. In very rare cases, some patients with significant corneal alteration (thin front layer of the eye) have developed white areas in the cornea due to calcium accumulation during treatment.
Reporting of suspected adverse reactions
This allows for continuous monitoring of the benefit/risk balance of the medicine. It is important to report any suspected adverse reactions to the medicine after authorization. Healthcare professionals are asked to report any suspected adverse reactions via the Spanish Medicines and Healthcare Products Agency's pharmacovigilance system for human use,
https://www.notificaRAM.es
5. Storage of REACTINE Levocabastina
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the container after EXP. The expiry date is the last day of the month indicated.
Discard the medicine 28 days after opening the container.
Store below 25°C.
Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition ofREACTINE Levocabastina
The active substance is Levocabastina.
Each milliliter contains 0.5 milligrams of levocabastina.
The other components (excipients) are: Benzalkonium chloride, Anhydrous disodium hydrogen phosphate, Sodium dihydrogen phosphate monohydrate, hypromellose, propylene glycol, polysorbate 80, disodium edetate, water for injectable preparations.
Appearance of the product and contents of the pack
REACTINE 0.5 mg/ml Levocabastina eye drops, suspension is packaged in boxes containing a 5 ml plastic bottle with 4 ml of homogeneous white suspension.
Marketing authorization holder
JNTL Consumer Health (Spain), S.L.
C/ Vía de los Poblados 1, Edificio E, planta 3
28033-Madrid
Spain
Manufacturer
FAMAR, S.A.
63, Agiou Dimitriou Str. (Alimos, Attica) - GR-17456 - Greece
Telephone: 302109898500 Telefax: 302109888800
Date of the last revision of this leaflet: February 2016
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REACTINE LEVOCABASTINE 0.5mg/ml EYE DROPS, SUSPENSIONDosage form: EYE DROP, 0.5 mg levocabastine / mlActive substance: levocabastineManufacturer: Esteve Pharmaceuticals S.A.Prescription requiredDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: olopatadineManufacturer: Tiedra Farmaceutica S.L.Prescription required
Online doctors for REACTINE LEVOCABASTINE 0.5mg/ml EYE DROPS, SUSPENSION
Discuss questions about REACTINE LEVOCABASTINE 0.5mg/ml EYE DROPS, SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















