
RATIOGRASTIM 30 MU/0.5 ml INJECTABLE SOLUTION OR FOR PERFUSION

How to use RATIOGRASTIM 30 MU/0.5 ml INJECTABLE SOLUTION OR FOR PERFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ratiograstim 30MU/0.5ml solution for injection or infusion
Ratiograstim 48MU/0.8ml solution for injection or infusion
Filgrastim
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Ratiograstim is and what it is used for
- What you need to know before you use Ratiograstim
- How to use Ratiograstim
- Possible side effects
- Storing Ratiograstim
- Contents of the pack and other information
- Information for self-injection
- This information is intended only for healthcare professionals
1. What Ratiograstim is and what it is used for
What Ratiograstim is
Ratiograstim contains the active substance filgrastim. Filgrastim is a protein produced by biotechnology in a bacterium called Escherichia coli.It belongs to a group of proteins called cytokines and is very similar to a natural protein produced by your own body - granulocyte colony-stimulating factor (GCSF).
Ratiograstim stimulates the bone marrow (the tissue where blood cells are made) to produce more blood cells, especially certain types of white blood cells. White blood cells are important because they help your body fight infections.
What Ratiograstim is used for
Your doctor has prescribed Ratiograstim to help your body produce more white blood cells. Your doctor will explain why you are being treated with Ratiograstim. Ratiograstim is useful in different situations such as:
- chemotherapy;
- bone marrow transplantation;
- severe chronic neutropenia (low white blood cell count);
- neutropenia in patients with HIV infection;
- mobilization of peripheral blood progenitor cells (for donation of blood progenitor cells).
2. What you need to know before you use Ratiograstim
Do not use Ratiograstim
- if you are allergic to filgrastim or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting treatment with Ratiograstim
- if you notice cough, fever, and difficulty breathing. This could be a consequence of a pulmonary disorder (see section 4. “Possible side effects”);
- if you have had sickle cell anemia (a hereditary disease characterized by sickle-shaped red blood cells).
- if you have pain in the upper left side of your abdomen or in your shoulder. This could be a consequence of a splenic disorder (see section 4. “Possible side effects”).
- if you have specific blood disorders (e.g. Kostman syndrome, myelodysplastic syndrome, or different types of leukemia).
- if you have osteoporosis. Your doctor may regularly check your bone density.
- if you have any other illness, especially if you think you have an infection.
Tell your doctor or nurse that you are being treated with Ratiograstim if you are undergoing
bone imaging tests.
During treatment with Ratiograstim, you will need to have regular blood tests to count the number of neutrophils and other white blood cells in your blood. This will tell your doctor how the treatment is working and whether you need to continue with it.
Using Ratiograstim with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Do not use Ratiograstim in the 24 hours before or after your chemotherapy treatment.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
The use of Ratiograstim in pregnant women has not been investigated. Therefore, your doctor will decide whether it is suitable for you not to use this medicine.
It is not known whether filgrastim passes into breast milk. Your doctor will decide whether it is suitable for you not to use this medicine while breastfeeding.
Driving and using machines
If you experience fatigue, do not drive or use tools or machines.
Ratiograstim contains sorbitol and sodium
If your doctor has told you that you have an intolerance to some sugars, consult them before using this medicine.
This medicine contains less than 1 mmol of sodium (23 mg) per pre-filled syringe; this is essentially “sodium-free”.
3. How to use Ratiograstim
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is…
The amount of Ratiograstim you need will depend on your illness and body weight. Your doctor will tell you when to stop treatment with Ratiograstim. It is quite normal to have several cycles of treatment.
Ratiograstim and chemotherapy
The usual dose is 0.5 million units (MU) per kilogram of body weight per day. For example, if you weigh 60 kg, your daily dose will be 30 million units (MU). You will usually receive your first dose 24 hours after the end of chemotherapy. Your treatment will usually last 14 days. However, in some types of diseases, a longer treatment with a duration of about a month may be necessary.
Ratiograstim and bone marrow transplantation
The usual initial dose is 1 million units (MU) per kilogram of body weight per day. For example, if you weigh 60 kg, your daily dose will be 60 million units (MU). You will usually receive your first dose 24 hours after the end of chemotherapy but within 24 hours of bone marrow transplantation. Your doctor will then perform daily blood tests to see how the treatment is working and find the most suitable dose for you. The treatment will be stopped when your white blood cells have reached a certain number in your blood.
Ratiograstim and severe chronic neutropenia
The usual initial dose is between 0.5 million units and 1.2 million units (MU) per kilogram of body weight per day as a single dose or divided into two doses. Your doctor will then perform blood tests to inform you about how the treatment is working and its duration. In the case of neutropenia, long-term treatment with Ratiograstim is required.
Ratiograstim and neutropenia in patients with HIV infection
The usual initial dose is between 0.1 million units and 0.4 million units (MU) per kilogram of body weight per day. Your doctor will then perform blood tests to inform you about how the treatment is working. Once your white blood cell count has returned to normal, the frequency of doses may be reduced to less than once a day. Your doctor will continue to perform blood tests and recommend the most suitable dose for you. To maintain a normal white blood cell count in your blood, long-term treatment with Ratiograstim may be necessary.
Ratiograstim and mobilization of peripheral blood progenitor cells
If you are your own donor of progenitor cells, the usual dose is 0.5 million units (MU) per kilogram of body weight per day. Treatment with Ratiograstim will last at least 2 weeks and in some cases will be longer. Your doctor will monitor your blood values to determine the best time to collect the progenitor cells.
If you are a donor of progenitor cells for another person, the usual dose is 1 million units (MU) per kilogram of body weight per day. Treatment with Ratiograstim will last between 4 and 5 days.
Method of administration
This medicine is administered by injection, either by intravenous (IV) infusion (a drip) or subcutaneous (SC) injection (into the tissue just under the skin). If you receive treatment by subcutaneous injection, your doctor may suggest that you learn to inject yourself. Your doctor or nurse will give you instructions on how to do this. Do not attempt to self-inject without these instructions. Part of the information you need appears at the end of this leaflet, but for optimal treatment of your illness, close and constant cooperation with your doctor is required.
If you use more Ratiograstim than you should
If you have used more Ratiograstim than you should, contact your doctor or pharmacist as soon as possible.
If you forget to use Ratiograstim
Do not use a double dose to make up for forgotten doses.
If you stop treatment with Ratiograstim
Before stopping treatment with Ratiograstim, consult your doctor.
If you have any further questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Important side effects
- Allergic reactions such as rash, inflamed skin with itching, and severe allergic reactions accompanied by weakness, low blood pressure, difficulty breathing, or swelling of the face have been reported. If you think you are having this type of reaction, you must stop administering Ratiograstim and seek immediate medical attention.
- Increased spleen size and spleen rupture have been reported. In some cases, spleen rupture was fatal. It is important that you consult your doctor immediately if you experience pain in the upper left side of your abdomen or in your left shoulder, as this may be related to a spleen problem.
- Cough, fever, difficulty breathing, and chest pain can be signs of a serious lung side effect, such as pneumonia or acute respiratory distress syndrome, which can be fatal. If you have a fever or any of these symptoms, seek immediate medical attention.
- Seek immediate medical attention if you experience any or a combination of the following side effects:
swelling that may be associated with urinating less frequently, difficulty breathing, abdominal swelling, and a general feeling of tiredness. These symptoms usually develop very quickly.
These may be symptoms of a rare disease (which can affect up to 1 in 100 people) called “capillary leak syndrome” and can cause blood to leak from small blood vessels into other parts of your body and require urgent medical attention.
- If you have sickle cell anemia, make sure to inform your doctor before starting treatment with Ratiograstim. Cases of sickle cell crisis have occurred in patients with sickle cell anemia who were using filgrastim.
- Filgrastim can cause muscle and bone pain as a very common side effect (which can affect more than 1 in 10 people). Ask your doctor what medicines you can take to help with this.
Additionally, you may experience the following side effects:
In patients with cancer
Very common(can affect more than 1 in 10 people):
- increase in some liver or blood enzymes; increase in uric acid in the blood;
- nausea; vomiting;
- chest pain.
Common(can affect up to 1 in 10 people):
- headache;
- cough; sore throat;
- constipation; loss of appetite; diarrhea; mucositis, which is a painful inflammation and ulcers in the lining of the digestive tract;
- hair loss; rash;
- fatigue; generalized weakness.
Uncommon(can affect up to 1 in 100 people):
- non-specific pain.
Rare(can affect up to 1 in 1,000 people):
- vascular disorders, which can cause pain, redness, and swelling of the limbs.
Very rare(can affect up to 1 in 10,000 people):
- painful, raised, purple lesions on the limbs, and sometimes on the face and neck, along with fever (Sweet's syndrome); inflammation of blood vessels, often with skin rash;
- worsening of rheumatic conditions;
- pain or difficulty urinating.
Frequency not known(frequency cannot be estimated from the available data):
- rejection of transplanted bone marrow;
- transient decrease in blood pressure;
- joint pain and swelling, similar to gout.
In healthy donors of hematopoietic progenitor cells
Very common(can affect more than 1 in 10 people):
- increase in white blood cell count; decrease in platelet count in the blood, which increases the risk of bleeding or bruising;
- headache.
Common(can affect up to 1 in 10 people):
- increase in some blood enzymes.
Uncommon(can affect up to 1 in 100 people):
- increase in some liver enzymes; increase in uric acid in the blood;
- worsening of rheumatic conditions.
Frequency not known(frequency cannot be estimated from the available data):
- cough; fever and difficulty breathing or coughing up blood.
In patients with severe chronic neutropenia
Very common(can affect more than 1 in 10 people):
- decrease in red blood cell count, which can make you pale and feel tired or short of breath;
- decrease in blood glucose levels; increase in some blood enzymes; increase in uric acid in the blood;
- nosebleeds.
Common(can affect up to 1 in 10 people):
- decrease in platelet count in the blood, which increases the risk of bleeding or bruising;
- headache;
- diarrhea;
- enlargement of the liver;
- hair loss; inflammation of blood vessels, often with skin rash; pain at the injection site; rash;
- bone loss of calcium; joint pain.
Uncommon(can affect up to 1 in 100 people):
- blood in the urine; protein in the urine.
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is not listed in this leaflet.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Annex V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Ratiograstim
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the pre-filled syringe after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Do not use this medicine if you notice turbidity or particles in the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Ratiograstim Composition
- The active ingredient is filgrastim. Each ml of injectable solution or infusion contains 60 million international units (MIU) (600 micrograms) of filgrastim.
Ratiograstim 30 MIU/0.5 ml: each pre-filled syringe contains 30 million international units (MIU) (300 micrograms) of filgrastim in 0.5 ml of solution.
Ratiograstim 48 MIU/0.8 ml: each pre-filled syringe contains 48 million international units (MIU) (480 micrograms) of filgrastim in 0.8 ml of solution.
- The other components are: sodium hydroxide, glacial acetic acid, sorbitol, polysorbate 80, water for injectable preparations.
Product Appearance and Container Contents
Ratiograstim is an injectable solution or infusion in a pre-filled syringe with or without a safety device. Ratiograstim is a clear and colorless solution. Each pre-filled syringe contains 0.5 ml or 0.8 ml of solution.
Ratiograstim is supplied in packs of 1, 5, or 10 pre-filled syringes or in multipacks containing 10 pre-filled syringes (2 packs of 5). Not all pack sizes may be marketed.
Marketing Authorization Holder
ratiopharm GmbH
Graf-Arco-Strasse 3
89079 Ulm
Germany
Manufacturer
Teva Biotech GmbH
Dornierstrasse 10
89079 Ulm
Germany
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Teva Pharma Belgium N.V./S.A./AG Tel: +32 3 820 73 73 | Lithuania UAB "Sicor Biotech" Tel: +370 5 266 0203 |
Bulgaria ???? ????????????? ???????? ???? Tel: +359 2 489 95 82 | Luxembourg ratiopharm GmbH Germany Tel: +49 731 402 02 |
Czech Republic Teva Pharmaceuticals CR, s.r.o. Tel: +420 251 007 111 | Hungary Teva Gyógyszergyár Zrt. Tel: +36 1 288 64 00 |
Denmark Teva Denmark A/S Tel: +45 44 98 55 11 | Malta Teva Pharmaceuticals Ireland Ireland Tel: +353 51 321740 |
Germany ratiopharm GmbH Tel: +49 731 402 02 | Netherlands Teva Nederland B.V. Tel: +31 800 0228 400 |
Estonia UAB "Sicor Biotech" Estonian branch Tel: +372 661 0801 | Norway Teva Norway AS Tel: +47 66 77 55 90 |
Greece Teva ?????? Α.Ε. Tel: +30 210 72 79 099 | Austria ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1 97 007 |
Spain Teva Pharma, S.L.U. Tel: +34 91 387 32 80 | Poland Teva Pharmaceuticals Polska Sp. z o.o. Tel: +48 22 345 93 00 |
France Teva Santé Tel: +33 1 55 91 78 00 | Portugal ratiopharm, Comércio e Indústria de Produtos Farmacêuticos, Lda Tel: +351 21 476 75 50 |
Croatia Pliva Hrvatska d.o.o. Tel: +385 1 37 20 000 | Romania Teva Pharmaceuticals S.R.L Tel: +40 21 230 65 24 |
Ireland Teva Pharmaceuticals Ireland Tel: +353 51 321740 | Slovenia Pliva Ljubljana d.o.o. Tel: +386 1 58 90 390 |
Iceland ratiopharm Oy, Finland Tel: +358 20 180 5900 | Slovakia TEVA Pharmaceuticals Slovakia s.r.o. Tel: +421 2 57 26 79 11 |
Italy Teva Italia S.r.l. Tel: +39 02 89 17 98 1 | Finland ratiopharm Oy Tel: +358 20 180 5900 |
Cyprus Teva ?????? Α.Ε., Greece Tel: +30 210 72 79 099 | Sweden Teva Sweden AB Tel: +46 42 12 11 00 |
Latvia UAB "Sicor Biotech" Latvian branch Tel: +371 673 23 666 | United Kingdom Teva UK Limited Tel: +44 1977 628500 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Information for Self-Injection
This section contains information on how to administer a Ratiograstim injection to yourself. It is important that you do not attempt to administer an injection without having received the necessary training from your doctor or nurse. If you are unsure about injecting yourself or have any doubts, consult your doctor or nurse.
How to Self-Inject Ratiograstim
You should inject into the tissue just under the skin. This is known as a subcutaneous injection. The injection should be administered once daily, approximately at the same time.
Equipment Needed for Administration
For subcutaneous injection, you will need:
- a pre-filled syringe of Ratiograstim,
- cotton or a similar material with alcohol.
What to Do Before Administering a Subcutaneous Injection of Ratiograstim
- Try to administer the injection at approximately the same time every day.
- Remove the pre-filled syringe of Ratiograstim from the refrigerator.
- Check the expiration date on the pre-filled syringe (EXP). Do not use it if the date is later than the last day of the month shown.
- Check the appearance of Ratiograstim. It should be a clear and colorless liquid. If there are particles inside, do not use it.
- To make the injection more comfortable, let the pre-filled syringe stand at room temperature for 30 minutes or gently roll it between your hands for a few minutes. Do not heat Ratiograstim in any other way (e.g., do not heat it in a microwave or in hot water).
- Do not remove the needle cap until you are ready to inject.
- Wash your hands carefully.
- Find a comfortable and well-lit place and put everything you need within reach (the pre-filled syringe of Ratiograstim and the cotton).
How to Prepare Your Ratiograstim Injection
Before injecting Ratiograstim, you must:
- Take the syringe and remove the protective cap from the needle gently without tilting it. Separate as indicated in images 1 and 2. Do not touch the needle or push the plunger.
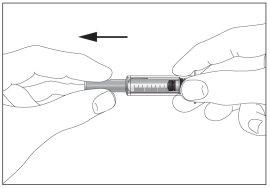 1
1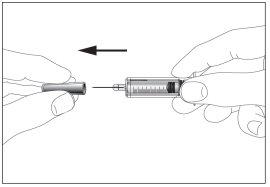 2
2
- A small air bubble may appear in the pre-filled syringe. If there are bubbles, tap the syringe gently with your fingers until the bubbles move to the end of the syringe. With the syringe pointing upwards, remove the air from the syringe by pushing the plunger.
- The syringe has a scale. Push the plunger until the number (ml) on the syringe corresponds to the prescribed dose of Ratiograstim.
- Check again that the dose of Ratiograstim is correct.
- Now you can use the pre-filled syringe.
Where to Inject
The most suitable injection sites are:
- the top of the thighs; and
- the abdomen, except for the area around the navel (see image 3).
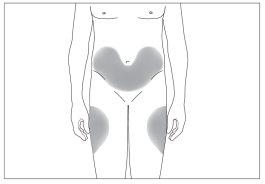 3
3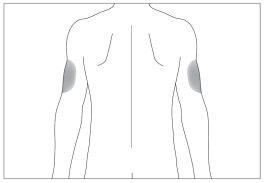 4
4
If someone else is administering the injection, they can also use the back of your upper arms (see image 4).
To avoid the risk of pain at a given point, it is best to change the injection site every day.
How to Inject
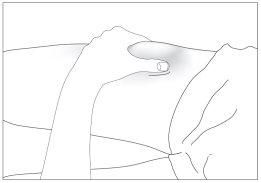
- Disinfect your skin using cotton with alcohol and pinch the skin between your thumb and index finger, without squeezing (see image 5).
- Insert the needle completely into the skin as your nurse or doctor has shown you (see image 6).
- Gently pull back the plunger to make sure you have not entered a vein. If you see blood in the syringe, remove the needle and insert it into another location.
- Inject the liquid slowly and regularly, always keeping the skin pinched.
- Inject only the dose prescribed by your doctor.
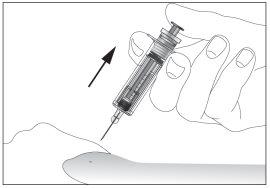
- Remove the needle from the injection site while keeping your finger on the plunger (see image 7). Point the needle away from you and those around you and activate the safety device by pressing the plunger firmly (see image 8). You will hear a "click", which confirms the activation of the safety device. The needle will be covered with a protective sheath so that you cannot prick yourself.
 5
5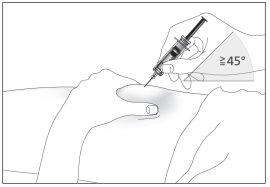 6
6
 7
7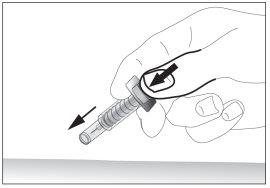 8
8
Remember
If you have any doubts, ask for help or advice from your doctor or nurse.
How to Dispose of Used Syringes
- The safety device prevents needlestick injuries after use, so no special disposal precautions are necessary. Dispose of the syringes with the safety device according to the instructions of your doctor, nurse, or pharmacist.
- This information is intended only for healthcare professionals.
Ratiograstim does not contain preservatives. Due to the potential risk of microbial contamination, Ratiograstim pre-filled syringes are for single use only.
Accidental exposure to freezing temperatures does not negatively affect the stability of Ratiograstim.
Ratiograstim should not be diluted with sodium chloride. This medicinal product should not be mixed with other medicinal products except those mentioned below. The dilution of filgrastim may be adsorbed by glass and plastic materials except if diluted as mentioned below.
Ratiograstim can be diluted, if necessary, in a 50 mg/ml (5%) glucose solution for infusion. It is not recommended to dilute to final concentrations below 0.2 MIU (2 μg) per ml. The solution should be inspected visually before use. Only clear solutions without particles should be used. In patients treated with filgrastim diluted to concentrations below 1.5 MIU (15 μg) per ml, human serum albumin (HSA) should be added to a final concentration of 2 mg/ml. Example: if the final injection volume is 20 ml and the total dose of filgrastim is below 30 MIU (300 μg), 0.2 ml of a 200 mg/ml (20%) human albumin solution should be administered. When Ratiograstim is diluted in a 50 mg/ml (5%) glucose solution for infusion, it is compatible with glass and various plastics such as PVC, polyolefin (copolymer of polypropylene and polyethylene), and polypropylene.
After dilution: the chemical and physical stability of the diluted infusion solution has been demonstrated for 24 hours stored between 2 and 8°C. From a microbiological point of view, the product should be used immediately. If not used immediately, the storage time and conditions of the diluted solution are the responsibility of the user and normally should not exceed 24 hours between 2 and 8°C, unless the dilution has been performed under validated and controlled aseptic conditions.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RATIOGRASTIM 30 MU/0.5 ml INJECTABLE SOLUTION OR FOR PERFUSIONDosage form: INJECTABLE, 12 IU/0.2 mlActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.3 mg / prefilled syringeActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.3 mg / prefilled syringeActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for RATIOGRASTIM 30 MU/0.5 ml INJECTABLE SOLUTION OR FOR PERFUSION
Discuss questions about RATIOGRASTIM 30 MU/0.5 ml INJECTABLE SOLUTION OR FOR PERFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






