How to use Neupogen
Leaflet attached to the packaging: patient information
Neupogen, 600 micrograms/ml (30 million IU/0.5 ml), solution for injection in a pre-filled syringe
Neupogen, 960 micrograms/ml (48 million IU/0.5 ml), solution for injection in a pre-filled syringe
filgrastim
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What Neupogen is and what it is used for
- 2. Important information before using Neupogen
- 3. How to use Neupogen
- 4. Possible side effects
- 5. How to store Neupogen
- 6. Contents of the pack and other information
- 7. Instructions for injecting Neupogen
1. What Neupogen is and what it is used for
Neupogen is a white blood cell growth factor (granulocyte-colony stimulating factor) belonging to a group of medicines called cytokines. Growth factors are proteins that are naturally produced in the body and can be produced and used as medicines using biotechnology methods.
Neupogen stimulates the bone marrow to produce more white blood cells.
A decrease in the number of white blood cells (neutropenia) can occur for several reasons. Neutropenia weakens the body's ability to fight infection. Neupogen stimulates the bone marrow to quickly produce new white blood cells.
Neupogen can be used:
- to increase the number of white blood cells after chemotherapy, to prevent the development of infections;
- to increase the number of white blood cells after bone marrow transplantation, to prevent the development of infections;
- before high-dose chemotherapy to stimulate the bone marrow to produce more stem cells, which can be harvested from the patient and then transplanted after the end of treatment. Stem cells can be harvested from the patient themselves or from a donor. Transplanted stem cells return to the bone marrow and produce blood cells;
- to increase the number of white blood cells in people with severe chronic neutropenia, to prevent the development of infections;
- in patients with advanced HIV infection to reduce the risk of developing infections.
2. Important information before using Neupogen
When not to use Neupogen
- if the patient is allergic to filgrastim or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Neupogen, discuss it with your doctor, pharmacist, or nurse.
Before starting treatment, inform your doctor if:
- sickle cell anemia has been diagnosed; Neupogen may exacerbate the disease.
- allergy to natural rubber (latex); the needle shield attached to the end of the pre-filled syringe is made of natural rubber and may cause an allergic reaction.
- osteoporosis (bone disease).
Tell your doctor immediately if you experience any of the following while using Neupogen:
- sudden symptoms of hypersensitivity, such as: rash, itching, swelling of the face, lips, tongue, or other parts of the body, shortness of breath, wheezing, or difficulty breathing, as these may be symptoms of a severe allergic reaction (hypersensitivity),
- swelling of the face or ankles, blood in the urine, or urine discolored to brown, or decreased urine output (kidney damage),
- pain in the upper left part of the abdomen, pain on the left side under the ribs, or pain at the top of the left shoulder [may be symptoms of spleen enlargement (splenomegaly) or possible spleen rupture],
- unusual bleeding or bruising [may be symptoms of decreased platelet count (thrombocytopenia) and decreased blood clotting ability],
- in patients with cancer and in healthy donors, rare cases of aortitis (inflammation of the aorta, a large blood vessel that carries blood from the heart to the rest of the body) have been observed. Symptoms may include: fever, abdominal pain, malaise, back pain, and increased levels of inflammatory markers. If you experience such symptoms, inform your doctor.
Loss of response to filgrastim
If the patient experiences a loss of response or inability to maintain a response to filgrastim treatment, the doctor will investigate the causes, including possible antibody production that neutralizes the effect of filgrastim.
The doctor may recommend closer monitoring of the patient, see section 4 of the leaflet.
In patients with severe chronic neutropenia, there is a risk of developing blood cancer [leukemia, myelodysplastic syndrome (MDS)]. Discuss with your doctor the risk of developing blood cancer and what tests should be performed. If you develop blood cancer or are at risk of developing it, do not use Neupogen unless your doctor recommends it.
Stem cell donors can only be individuals between 16 and 60 years old.
Exercise special caution when using other white blood cell-stimulating products
Neupogen belongs to a group of medicines that stimulate the production of white blood cells. The treating doctor should always clearly record the trade name of the medicine being administered to the patient.
Neupogen and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
Pregnancy and breastfeeding
No studies have been conducted with Neupogen in pregnant or breastfeeding women. Neupogen should not be used during pregnancy.
It is essential to inform your doctor:
- if you are pregnant or breastfeeding,
- if you think you may be pregnant; or
- if you plan to have a baby. If you become pregnant while using Neupogen, inform your doctor.
If your doctor does not recommend otherwise, you should stop breastfeeding while using Neupogen.
Driving and using machines
Neupogen may have a minor influence on the ability to drive and use machines. This medicine may cause dizziness. It is recommended that the patient wait and see how they feel after taking Neupogen before driving or operating machinery.
Neupogen contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per pre-filled syringe, which means the medicine is considered 'sodium-free'.
Neupogen contains sorbitol
The medicine contains 50 mg of sorbitol per ml.
Sorbitol is a source of fructose. If you have hereditary fructose intolerance, a rare genetic disorder, you should not take this medicine. In people with hereditary fructose intolerance, the body does not break down the fructose contained in this medicine, which can cause serious side effects.
Tell your doctor before taking this medicine if you have hereditary fructose intolerance or if you cannot tolerate sweet foods or drinks due to nausea, vomiting, or unpleasant side effects such as bloating, stomach cramps, or diarrhea.
3. How to use Neupogen
This medicine should always be used exactly as your doctor has told you. If you are not sure, ask your doctor, nurse, or pharmacist.
How Neupogen is given and what dose is used
Neupogen is usually given daily by injection under the skin (subcutaneous injection). The medicine can also be given daily by slow injection into a vein (intravenous infusion). The dose depends on the type of disease and the patient's body weight.
Your doctor will tell you what dose to use.
Patient undergoing bone marrow transplantation after chemotherapy:
Usually, the patient will receive the first dose of Neupogen at least 24 hours after chemotherapy or at least 24 hours after bone marrow transplantation.
The patient or their caregivers may be trained to perform subcutaneous injections to continue treatment at home. The patient should not attempt to do this unless they have been properly trained by their doctor.
How long to use Neupogen
Neupogen should be used until the white blood cell count returns to normal. Regular blood tests will be performed to check the white blood cell count in the body. Your doctor will tell you how long to use Neupogen.
Use in children
Neupogen is used in children receiving chemotherapy or suffering from severe chronic neutropenia (significant decrease in white blood cell count). The dosing in children receiving chemotherapy is the same as in adults.
Using a higher dose of Neupogen than recommended
Do not exceed the dose recommended by your doctor. If you suspect that you have used a higher dose than recommended, contact your doctor as soon as possible.
Missing a dose of Neupogen
If you miss a dose or use a lower dose than recommended, inform your doctor as soon as possible. Do not use a double dose to make up for a missed dose.
If you have any further questions about using this medicine, ask your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediatelyif you experience any of the following during treatment:
- an allergic reaction, including weakness, sudden drop in blood pressure, difficulty breathing, swelling of the face (anaphylaxis), skin rash, itching skin or hives (urticaria), swelling of the face, lips, mouth, tongue, or throat (angioedema), and shortness of breath (dyspnea).
- cough, fever, and breathing problems (dyspnea), as these may be symptoms of acute respiratory distress syndrome.
- kidney damage (renal failure). Kidney damage has occurred in patients receiving Neupogen. Contact your doctor immediately if you experience swelling of the face or ankles, blood in the urine, or urine discolored to brown, or decreased urine output.
- any of the following side effects or a combination of them:
- swelling or edema, which may be associated with less frequent urination, difficulty breathing, abdominal swelling, or a feeling of fullness and general fatigue. These symptoms usually develop rapidly.
These may be symptoms of a condition called "capillary leak syndrome", which causes blood to leak from small blood vessels into the body and requires immediate medical attention.
- any of the following symptoms:
- fever or chills or a feeling of intense cold, rapid heart rate, confusion, disorientation, shortness of breath, severe pain or discomfort, and sweaty or clammy skin. These may be symptoms of sepsis (blood poisoning), a serious infection that can be life-threatening and requires immediate medical attention.
- pain in the upper left part of the abdomen, pain on the left side under the ribs, or pain at the top of the left shoulder, as these may indicate spleen problems [enlargement of the spleen (splenomegaly) or possible spleen rupture].
- blood in the urine (hematuria) in patients with severe chronic neutropenia. Your doctor may recommend regular urine tests if you experience this side effect or if protein is present in the urine (proteinuria).
A common side effect of Neupogen is muscle or bone pain (musculoskeletal pain), which can be relieved by taking commonly used painkillers. In patients undergoing stem cell or bone marrow transplantation, graft-versus-host disease may occur - this is a reaction of the donor cells against the recipient's body; symptoms and signs of this disease include rash on the palms of the hands or soles of the feet, and ulcers and pain in the mouth, gut, liver, skin, or eyes, lungs, vagina, and joints.
In healthy stem cell donors, increased white blood cell count (leukocytosis) and decreased platelet count (thrombocytopenia) have been observed, which will be monitored by your doctor.
Very common side effects(may affect more than 1 in 10 people):
- decreased platelet count, which reduces the ability of blood to clot (thrombocytopenia)
- decreased red blood cell count (anemia)
- headache
- diarrhea
- vomiting
- nausea
- abnormal hair loss or thinning (alopecia)
- fatigue
- pain and swelling of the mucous membranes of the digestive tract from the mouth to the anus (mucositis)
- fever
Common side effects(may affect up to 1 in 10 people):
- pneumonia (bronchitis)
- upper respiratory tract infection
- urinary tract infection
- decreased appetite
- sleep disorders (insomnia)
- dizziness
- numbness or tingling, especially in the skin (paresthesia)
- low blood pressure (hypotension)
- high blood pressure (hypertension)
- cough
- hemoptysis (coughing up blood)
- mouth and throat pain (oropharyngeal pain)
- nasal bleeding
- constipation
- mouth pain
- enlarged liver (hepatomegaly)
- rash
- redness of the skin (erythema)
- muscle spasms
- pain when urinating (dysuria)
- chest pain
- general discomfort
- general weakness (asthenia)
- general feeling of being unwell
- swelling of the hands and feet (peripheral edema)
- increased activity of some enzymes in the blood
- changes in blood biochemistry test results
- transfusion reaction Uncommon side effects(may affect up to 1 in 100 people):
- increased white blood cell count (leukocytosis)
- allergic reaction (hypersensitivity)
- graft-versus-host disease (graft-versus-host disease)
- high levels of uric acid in the blood, which can cause gout (hyperuricemia)
- liver damage caused by blockage of small veins in the liver (hepatic veno-occlusive disease)
- abnormal lung function leading to shortness of breath (respiratory failure)
- swelling and fluid in the lungs (pulmonary edema)
- inflammation of the lung tissue (interstitial lung disease)
- abnormal lung image on X-ray (pulmonary infiltrates)
- bleeding from the lungs (pulmonary hemorrhage)
- oxygen uptake problems in the lungs (hypoxemia)
- elevated skin rash (maculopapular rash)
- bone disease that causes bone thinning, weakening, and fragility (osteoporosis)
- reaction at the injection site
Rare side effects(may affect up to 1 in 1,000 people):
- severe bone pain, chest pain, abdominal pain, or joint pain (sickle cell crisis with pain)
- severe, life-threatening allergic reaction (anaphylactic reaction)
- joint pain and swelling similar to gout (pseudogout)
- fluid regulation disorder that can cause swelling (fluid volume disorder)
- inflammation of the blood vessels in the skin
- purple, raised, painful skin ulcers on the limbs, sometimes on the face and neck, with fever (Sweet's syndrome)
- worsening of rheumatoid arthritis symptoms
- abnormal urine changes
- decreased bone mineral density
- aortic inflammation (aortitis), see section 2
- blood cell production outside the bone marrow (extramedullary hematopoiesis)
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or nurse. Side effects can be reported directly to:
Department for Monitoring of Adverse Reactions to Medicinal Products, Medical Devices, and Biocides
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Neupogen
Keep this medicine out of the sight and reach of children.
Store in a refrigerator (2°C – 8°C).
Store the pre-filled syringe in the outer carton to protect from light.
Accidental freezing does not affect the quality of the medicine.
Do not use this medicine after the expiry date stated on the label and carton after: Expiry date or EXP. The expiry date refers to the last day of the month stated.
Do not use this medicine if you notice discoloration, cloudiness, or particles. The solution should be clear and colorless.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Neupogen contains
- The active substance is filgrastim at a strength of 600 micrograms/ml (30 million IU/0.5 ml) or 960 micrograms/ml (48 million IU/0.5 ml).
- The other ingredients are: acetic acid, sodium hydroxide 1N, sorbitol (E420), polysorbate 80, water for injections.
What Neupogen looks like and contents of the pack
Neupogen is a clear, colorless solution for injection (injection)/concentrate for solution for infusion (sterile concentrate) in a pre-filled syringe.
Neupogen is available in packs containing one pre-filled syringe.
Marketing authorization holder and manufacturer
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Marketing authorization holder
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Manufacturer
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co Dublin
Ireland
Manufacturer
Amgen NV
Telecomlaan 5-7
1831 Diegem
Belgium
To obtain more detailed information on this medicine, contact the local representative of the marketing authorization holder:
Poland
Amgen Biotechnology Sp. z o.o.
ul. Puławska 145
02-715 Warsaw
Phone: +48 22 581 3000
[email protected]
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the name
Neupogen, except in Cyprus, Greece, and Italy, where it is marketed under the name Granulokine.
Date of last revision of the leaflet: March 2024.
Other sources of information
Detailed information on this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocides (www.urpl.gov.pl/).
---------------------------------------------------------------------------------------------------------------------------
7. Instructions for injecting Neupogen
This section provides information on how to inject Neupogen.
Important:do not attempt to inject Neupogen yourself unless you have been trained by your doctor or nursing staff.
Neupogen injection is given under the skin. This method of giving medicines is called a subcutaneous injection.
Equipment needed to administer the medicine
To inject Neupogen yourself, you will need:
- a Neupogen pre-filled syringe,
- alcohol swabs or similar.
What to do before injecting Neupogen yourself
- 1. Remove one tray from the refrigerator and let it stand at room temperature for about 30 minutes or hold it gently in your hand for a few minutes. This will make the injection less painful. Do notheat Neupogen in any other way (e.g., in a microwave or hot water).
- 2. Do not shake the pre-filled syringe.
- 3. Place the tray in your hand and remove the paper cover.
- 4. Turn the tray over and place the pre-filled syringe in your hand.
- 5. Do notremove the needle cover until you are ready to inject.
- 6. Check the expiry date on the label of the pre-filled syringe (EXP). Do not use the medicine if the last day of the month stated on the label has passed.
- 7. Check the appearance of the medicine. Neupogen should be a clear and colorless liquid. Do not use the medicine if you notice discoloration, cloudiness, or particles.
- 8.
Wash your hands carefully.
- 9. Gather the necessary items in a clean, easily accessible, and well-lit area.
How to prepare the Neupogen injection
Before injecting Neupogen, you should:
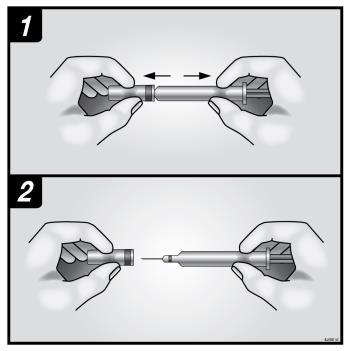
- 1. To avoid bending the needle, hold the glass cylinder of the pre-filled syringe safely; gently, without twisting, remove the needle cover, as shown in figures 1 and 2.
- 2. Do not touch the needle or press the plunger.
- 3. Small air bubbles may be visible in the pre-filled syringe. Removing these air bubbles before injection is not necessary. Injecting the solution containing air bubbles is harmless.
- 4. The pre-filled syringe is now ready for use.
Where to inject Neupogen yourself
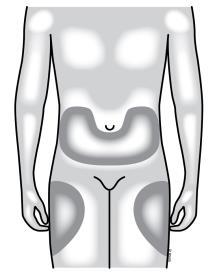
The best places to inject yourself are the upper thighs and abdomen. If someone else is injecting, they can use the back of the arms.
You can change the injection site if the current site becomes red or sore.
How to inject Neupogen yourself
- 1. Disinfect the skin with an alcohol swab and grasp (but do not squeeze) the skin fold between your thumb and index finger.
- 2. Insert the needle all the way into the skin, as shown by your nurse or doctor.
- 3. Push the plunger with steady pressure, keeping the skin fold between your fingers, and inject the medicine until the pre-filled syringe is empty.
- 4. Remove the needle and release the skin fold.
- 5. If a drop of blood appears at the injection site, gently wipe it off with a swab or cotton ball. Do not rub the skin at the injection site. If necessary, you can apply a plaster to the injection site.
- 6. One pre-filled syringe is for single use only. Do not use Neupogen that has been left in the pre-filled syringe.
Important:if you have any doubts, do not hesitate to ask your doctor or nursing staff for help.
Disposal of used syringes
- Do not replace the needle cover on used needles, as this may cause accidental needlestick injury.
- Used syringes should be stored in a place that is inaccessible and invisible to children.
- Used syringes should not be disposed of in household waste. Ask your pharmacist what to do with used syringes or those that are no longer needed.
Information intended for healthcare professionals only:
When used as a concentrate for solution for infusion, Neupogen should be diluted in 20 ml of 5% glucose solution. Detailed information is available in the Summary of Product Characteristics.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAmgen Europe B.V. Amgen NV Amgen Technology (Ireland) Unlimited Company
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NeupogenDosage form: Solution, 300 mcg/ml (30 million IU/ml)Active substance: filgrastimPrescription not requiredDosage form: Solution, 960 mcg/ml (48 million IU/0.5 ml)Active substance: filgrastimPrescription not requiredDosage form: Powder, min. 2x10^8 max. 3x10^9/50 mlActive substance: BCG vaccinePrescription required
Alternatives to Neupogen in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Neupogen in Spain
Alternative to Neupogen in Ukraine
Online doctors for Neupogen
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Neupogen – subject to medical assessment and local rules.