QDENGA powder and solvent for injectable solution in pre-filled syringe

How to use QDENGA powder and solvent for injectable solution in pre-filled syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Qdenga Powder and Solvent for Solution for Injection in Pre-filled Syringe
Tetravalent Dengue Vaccine (Live, Attenuated)
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you or your child are vaccinated because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you or your child only. Do not pass it on to others.
- If you or your child get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Pack
- What Qdenga is and what it is used for
- What you need to know before you or your child receive Qdenga
- How Qdenga is administered
- Possible side effects
- Storage of Qdenga
- Contents of the pack and other information
1. What Qdenga is and what it is used for
Qdenga is a vaccine. It is used to help protect you or your child against dengue. Dengue is a disease caused by serotypes 1, 2, 3, and 4 of the dengue virus. Qdenga contains weakened versions of these 4 serotypes of the dengue virus, so it cannot cause the disease.
Qdenga is administered to adults, adolescents, and children (from 4 years of age).
Qdenga should be used in accordance with official recommendations.
How the vaccine works
Qdenga stimulates the body's natural defenses (immune system). This helps protect against the viruses that cause dengue if the body is exposed to these viruses in the future.
What is dengue
Dengue is caused by a virus.
- The virus is transmitted through mosquitoes (Aedes mosquitoes).
- If a mosquito bites a person with dengue, it can transmit the virus to the next people it bites.
Dengue is not transmitted directly from person to person.
The signs of dengue include fever, headache, pain behind the eyes, muscle and joint pain, sensitivity or discomfort (nausea and vomiting), swelling of the lymph nodes or skin rash. The signs of dengue usually last from 2 to 7 days. You or your child may also be infected with the dengue virus but not show signs of illness.
Occasionally, dengue can be severe enough for you or your child to need hospitalization, and in rare cases, it can cause death. Severe dengue can cause high fever and any of the following symptoms: severe abdominal pain, persistent nausea (vomiting), rapid breathing, severe bleeding, stomach bleeding, gum bleeding, fatigue, restlessness, coma, seizures, and organ dysfunction.
2. What you need to know before you or your child receive Qdenga
To ensure that Qdenga is suitable for you or your child, it is important that you inform your doctor, pharmacist, or nurse if any of the following points apply to you or your child. If there is anything you do not understand, ask your doctor, pharmacist, or nurse to explain.
Do not use Qdenga if you or your child
- are allergic to the active substances or any of the other ingredients of Qdenga (listed in section 6).
- have had an allergic reaction after receiving Qdenga in the past. The signs of an allergic reaction can include an itchy rash, difficulty breathing, and swelling of the face and tongue.
- have a weakened immune system (the body's natural defenses). This can be due to a genetic defect or an HIV infection.
- are taking a medicine that affects the immune system (such as high doses of corticosteroids or chemotherapy). Your doctor will not use Qdenga until 4 weeks after you stop treatment with this medicine.
- are pregnant or breastfeeding.
Do not use Qdenga if any of the above apply.
Warnings and precautions
- Tell your doctor, pharmacist, or nurse before receiving Qdenga if you or your child:
- have a feverish infection. Vaccination may need to be postponed until recovery.
- have ever had health problems when given a vaccine. Your doctor will carefully weigh the risks and benefits of vaccination.
- have ever fainted after an injection. Fainting, dizziness, and occasionally falls (especially in young people) can occur after or even before any injection with a needle.
Important information about the protection provided
As with any vaccine, Qdenga may not protect all those who receive it, and protection may decrease over time. You or your child can still get dengue from mosquito bites, including severe dengue. You or your child should continue to protect themselves against mosquito bites even after vaccination with Qdenga.
After vaccination, you or your child should consult a doctor if you or your child think you or your child may have a dengue infection and have any of the following symptoms: high fever, severe abdominal pain, persistent vomiting, rapid breathing, bleeding gums, fatigue, restlessness, and blood in the vomit.
Other protective measures
You or your child should take precautions to avoid mosquito bites. This includes using insect repellents, protective clothing, and mosquito nets.
Young children
Children under 4 years of age should not receive Qdenga.
Other medicines and Qdenga
Qdenga can be administered with the hepatitis A vaccine, the yellow fever vaccine, or the human papillomavirus vaccine at a different injection site (a different part of the body, usually the other arm) during the same visit.
Tell your doctor or pharmacist if you or your child are using, have recently used, or might use any other medicine or vaccine.
In particular, tell your doctor or pharmacist if you or your child are taking any of the following substances:
- Medicines that affect the body's natural defenses (immune system) such as high doses of corticosteroids or chemotherapy. In this case, your doctor will not use Qdenga until 4 weeks after you stop treatment. This is because Qdenga may not work as well.
- Medicines called "immunoglobulins" or blood products that contain immunoglobulins, such as blood or plasma. In this case, your doctor will not use Qdenga until 6 weeks and preferably 3 months after you stop treatment. This is because Qdenga may not work as well.
Pregnancy and breastfeeding
Do not use Qdenga if you or your daughter are pregnant or breastfeeding. If you or your daughter:
- are of childbearing potential, you or your daughter should take precautions to avoid becoming pregnant for a month after vaccination with Qdenga.
- think you or your daughter might be pregnant or are planning to have a baby, ask your doctor, pharmacist, or nurse for advice before using Qdenga.
Driving and using machines
Qdenga has a minor influence on the ability to drive and use machines in the first few days after vaccination.
Qdenga contains sodium and potassium
This medicine contains less than 1 mmol of sodium (23 mg) per 0.5 ml dose; i.e., it is essentially "sodium-free".
This medicine contains less than 1 mmol (39 mg) of potassium per 0.5 ml dose; i.e., it is essentially "potassium-free".
3. How Qdenga is administered
Qdenga is administered by a doctor or nurse as an injection under the skin (subcutaneous injection) in the upper arm. It must not be injected into a blood vessel.
You or your child will receive 2 injections.
The second injection is administered 3 months after the first.
There are no data in adults over 60 years of age. Consult your doctor to see if Qdenga is beneficial for you.
Qdenga should be used in accordance with official recommendations.
Instructions for preparation of the vaccine for doctors and healthcare professionals are at the end of the leaflet.
If you or your child miss a Qdenga injection
- If you or your child miss a scheduled injection, the doctor will decide when to administer the missed injection. It is important that you or your child follow the doctor's, pharmacist's, or nurse's instructions regarding the follow-up injection.
- If you forget or are unable to return at the scheduled time, ask your doctor, pharmacist, or nurse for advice.
If you have any further questions on the use of this vaccine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Qdenga can cause side effects, although not everybody gets them.
Severe allergic reaction (anaphylactic)
If any of the following symptoms occur after leaving the place where you or your child received an injection, contact a doctor immediately:
- difficulty breathing
- blue-tinged lips or tongue
- rash
- swelling of the face or throat
- low blood pressure that causes dizziness or fainting
- sudden and severe feeling of discomfort or restlessness, accompanied by a drop in blood pressure that causes dizziness and loss of consciousness, as well as rapid heartbeat associated with difficulty breathing
These signs or symptoms (anaphylactic reactions) usually occur soon after administration of the injection and while you or your child are still in the medical center or doctor's office. They can also occur very rarely after receiving any vaccine.
The following side effects have occurred during studies in children, adolescents, and adults.
Very common(may affect more than 1 in 10 people):
- pain at the injection site
- headache
- muscle pain
- redness at the injection site
- feeling unwell
- weakness
- infections of the nose or throat
- fever
Common(may affect up to 1 in 10 people):
- swelling at the injection site
- pain or swelling of the nose or throat
- bruising at the injection site
- itching at the injection site
- swelling of the throat and tonsils
- joint pain
- flu-like illness
Uncommon(may affect up to 1 in 100 people):
- diarrhea
- nausea
- stomach pain
- vomiting
- bleeding at the injection site
- feeling dizzy
- itching of the skin
- skin rash, including skin rashes with spots or itching of the skin
- hives
- fatigue
- change in skin color at the injection site
- inflammation of the airways
- runny nose
Rare(may affect up to 1 in 10,000 people):
- rapid swelling under the skin in areas such as the face, throat, arms, and legs
Frequency not known(cannot be estimated from the available data):
- sudden and severe allergic reaction (anaphylactic) with difficulty breathing, swelling, fainting, rapid heartbeat, sweating, and loss of consciousness
Other side effects in children from 4 to 5 years of age:
Very common(may affect more than 1 in 10 people):
- decreased appetite
- feeling sleepy
- irritability
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Qdenga
Keep Qdenga out of the sight and reach of children.
Do not use Qdenga after the expiry date stated on the carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Store the vaccine in the outer packaging.
After mixing (reconstitution) with the supplied solvent, Qdenga should be used immediately. If not used immediately, Qdenga must be used within 2 hours.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and additional information
What Qdenga contains
- After reconstitution, one dose (0.5 ml) contains:
Dengue virus serotype 1 (live, attenuated)*: ≥ 3.3 log10 PFU**/dose
Dengue virus serotype 2 (live, attenuated)#: ≥ 2.7 log10 PFU**/dose
Dengue virus serotype 3 (live, attenuated)*: ≥ 4.0 log10 PFU**/dose
Dengue virus serotype 4 (live, attenuated)*: ≥ 4.5 log10 PFU**/dose
- Produced in Vero cells using recombinant DNA technology. Genes of the specific surface proteins of each serotype, inserted into the skeleton of dengue type 2. This product contains genetically modified organisms (GMOs).
# Produced in Vero cells by recombinant DNA technology.
** PFU = plaque-forming units
- The other ingredients are: a,a-trehalose dihydrate, poloxamer 407, human serum albumin, potassium dihydrogen phosphate, sodium dihydrogen phosphate, potassium chloride, sodium chloride, water for injectable preparations.
Appearance of Qdenga and pack contents
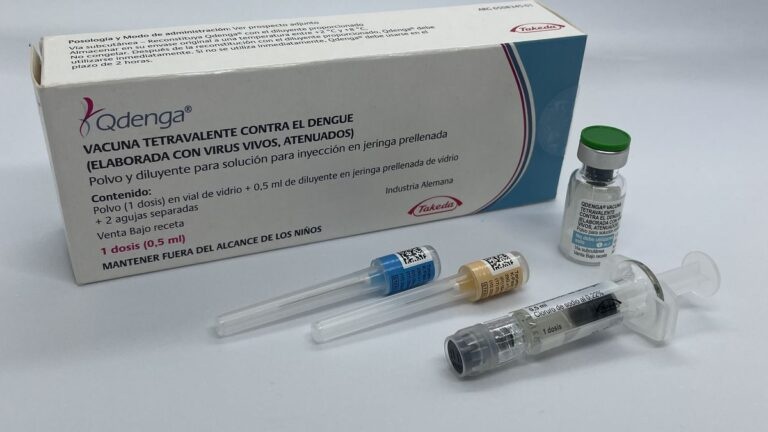
Qdenga is a powder and solvent for solution for injection. Qdenga is supplied as a powder in a single-dose vial and a solvent in a pre-filled syringe with 2 separate needles or without a needle.
The powder and solvent must be mixed before use.
Qdenga powder and solvent for solution for injection in a pre-filled syringe are available in packs of 1 or 5.
Only certain pack sizes may be marketed.
The powder is a compact white to off-white suspension.
The solvent (0.22% sodium chloride solution) is a colorless and transparent liquid.
After reconstitution, Qdenga is a transparent solution, colorless to pale yellow, essentially free from foreign particles.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Takeda GmbH
Byk-Gulden-Str. 2
78467 Konstanz
Germany
Manufacturer
Takeda GmbH
Production site Singen
Robert-Bosch-Str. 8
78224 Singen
Germany
For further information on this medicinal product, please contact the local representative of the marketing authorisation holder:
België/Belgique/Belgien Takeda Belgium NV Tél/Tel: +32 2 464 06 11 | Lietuva Takeda, UAB Tel: +370 521 09 070 |
| Luxembourg/Luxemburg Takeda Belgium NV Tél/Tel: +32 2 464 06 11 |
Ceská republika Takeda Pharmaceuticals Czech Republic s.r.o. Tel: +420 234 722 722 | Magyarország Takeda Pharma Kft. Tel: +36 1 270 7030 |
Danmark Takeda Pharma A/S Tlf.: +45 46 77 10 10 | Malta Takeda HELLAS S.A. Tel: +30 210 6387800 |
Deutschland Takeda GmbH Tel: +49 (0) 800 825 3325 | Nederland Takeda Nederland B.V. Tel: +31 20 203 5492 |
Eesti Takeda Pharma AS Tel: +372 6177 669 | Norge Takeda AS Tlf: 800 800 30 |
Ελλáδα Takeda ΕΛΛΑΣ Α.Ε. Τηλ: +30 210 6387800 | Österreich Takeda Pharma Ges.m.b.H. Tel: +43 (0) 800-20 80 50 |
España Takeda Farmacéutica España S.A. Tel: +34 917 90 42 22 | Polska Takeda Pharma sp. z o.o. Tel: +48 22 306 24 47 |
Francia Takeda France SAS Tél: +33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel: +351 21 120 1457 |
Hrvatska Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | Ireland Takeda Products Ireland Ltd. Tel: 1800 937 970 |
România Takeda Pharmaceuticals SRL Tel: +40 21 335 03 91 | Slovenija Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel: +386 (0) 59 082 480 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Takeda Pharmaceuticals Slovakia s.r.o. Tel: +421 (2) 20 602 600 |
Italia Takeda Italia S.p.A. Tel: +39 06 502601 | Suomi/Finland Takeda Oy Puh/Tel: 0800 774 051 |
Κúπρος Takeda ΕΛΛΑΣ Α.Ε. Τηλ: +30 2106387800 | Sverige Takeda Pharma AB Tel: 020 795 079 |
Latvija Takeda Latvia SIA Tel: +371 67840082 | United Kingdom (Northern Ireland) Takeda UK Ltd Tel: +44 (0) 3333 000 181 |
Date of last revision of this leaflet: October 2024.
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
This information is intended for healthcare professionals only:
- Qdenga must not be mixed with other medicinal products or vaccines in the same syringe.
- Qdenga must not be administered as an intravascular injection under any circumstances.
- Vaccination should be performed by subcutaneous injection, preferably in the upper arm, in the deltoid. Qdenga must not be administered as an intramuscular injection.
- Syncope (fainting) may occur after, or even before, any vaccination as a psychogenic response to the needle injection. Procedures should be in place to avoid injury from fainting and manage syncopal reactions.
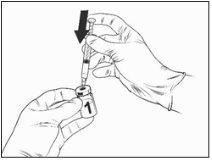
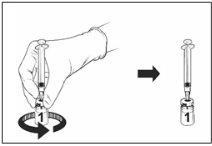
Instructions for reconstitution of the vaccine with the solvent presented in the pre-filled syringe:
Qdenga is a 2-component vaccine consisting of a vial containing the lyophilized vaccine and solvent supplied in the pre-filled syringe. The lyophilized vaccine must be reconstituted with the solvent before administration.
Qdenga must not be mixed with other vaccines in the same syringe.
To reconstitute Qdenga, use only the solvent (0.22% sodium chloride solution) in the pre-filled syringe supplied with the vaccine, as it does not contain preservatives or other antiviral substances. Contact with preservatives, antiseptics, detergents, and other antiviral substances should be avoided, as they may inactivate the vaccine.
Remove the vaccine vial and solvent from the pre-filled syringe from the refrigerator and let them stand at room temperature for approximately 15 minutes.
Vaccine vial |
|
Reconstituted vaccine |
|
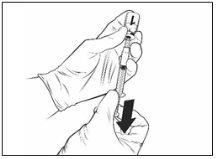
After reconstitution, the resulting solution should be transparent, colorless to pale yellow, and essentially free from foreign particles. Discard the vaccine if particles or color changes are observed.
Reconstituted vaccine |
|
Qdenga should be administered immediately after reconstitution. Its physicochemical stability has been demonstrated during use for a period of 2 hours at room temperature (up to 32.5°C) from the time of reconstitution of the vaccine vial. After this period, the vaccine should be discarded. Do not put it back in the refrigerator. From a microbiological point of view, Qdenga should be used immediately. If not used immediately, the storage times and conditions will be the responsibility of the user.
Disposal of unused medicinal products and all waste materials should be done in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to QDENGA powder and solvent for injectable solution in pre-filled syringeDosage form: INJECTABLE, 60 micrograms/dose + 60 micrograms/doseActive substance: respiratory syncytial virus vaccinesManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 0.5 mLActive substance: respiratory syncytial virus vaccinesManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 50 µgActive substance: respiratory syncytial virus vaccinesManufacturer: Moderna Biotech Spain S.L.Prescription required
Online doctors for QDENGA powder and solvent for injectable solution in pre-filled syringe
Discuss questions about QDENGA powder and solvent for injectable solution in pre-filled syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions