
QALSODY 100 mg INJECTABLE SOLUTION

How to use QALSODY 100 mg INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
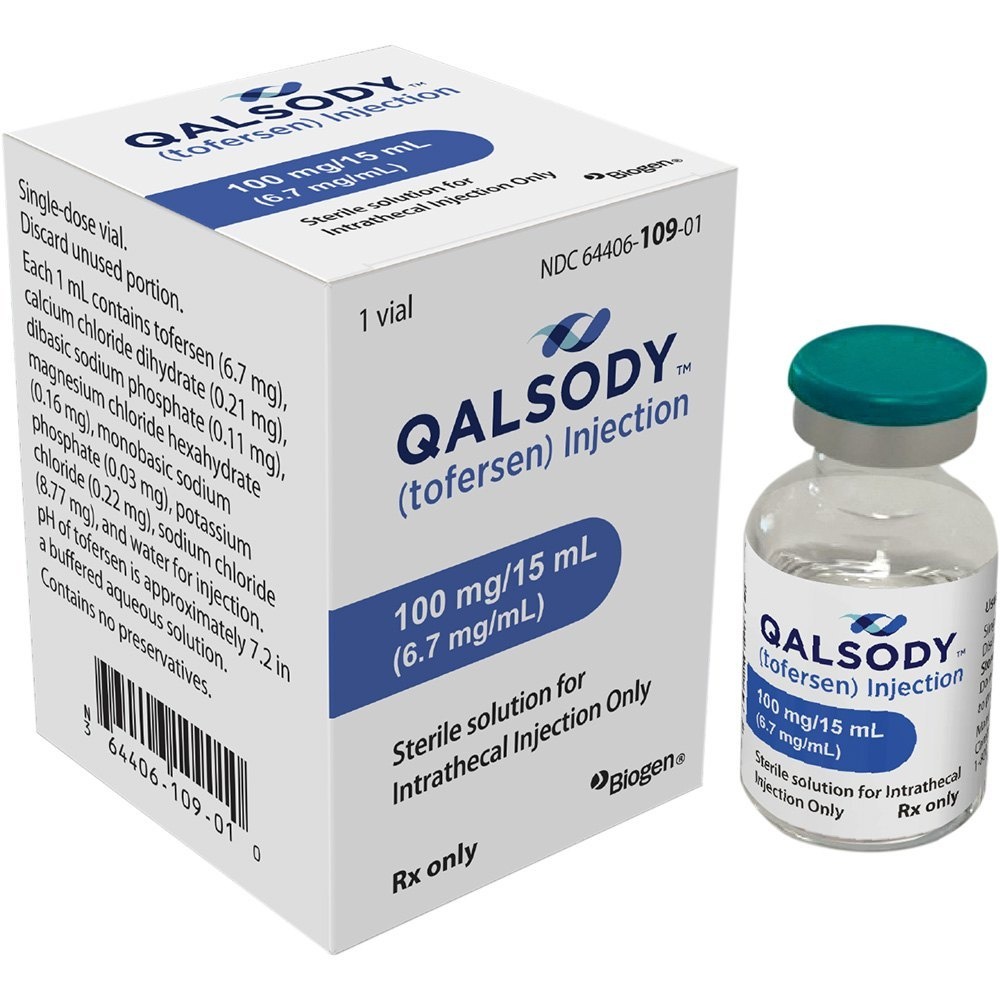
Qalsody 100 mg solution for injection
tofersen
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Qalsody and what is it used for
- What you need to know before you are given Qalsody
- How Qalsody is given
- Possible side effects
- Storage of Qalsody
- Contents of the pack and other information
1. What is Qalsody and what is it used for
Qalsody contains the active substance tofersen, which belongs to a group of medicines known as antisense oligonucleotides.
This medicine is used in adults to treat a type of amyotrophic lateral sclerosis(ALS) caused by mutations (changes) in a gene called SOD1.
The ALS caused by mutations in the SOD1 gene is a rare type of motor neuron disease that affects the nerve cells in the brain and spinal cord. The mutations in the SOD1 gene produce an accumulation of a toxic form of the SOD1 protein. This causes the destruction of motor neurons (the nerve cells responsible for sending instructions to the muscle), leading to muscle weakness and atrophy, including those used for breathing and swallowing.
Qalsody reduces the accumulation of the SOD1 protein. This helps to prevent the destruction of motor neurons and may slow down the loss of muscle strength.
2. What you need to know before you are given Qalsody
Qalsody must not be given
- if you are allergic to tofersenor to any of the other ingredients of this medicine (listed in section 6).
Talk to your doctor or nursebefore starting treatment if this applies to you.
Warnings and precautions
There is a risk that side effects may occur after the administration of Qalsody by the lumbar puncture procedure (see section 3). These may include headache, back pain, and infection.
A small number of cases of patients who have developed inflammation of the spinal cord (myelitis) or irritation or injury of the nerve roots (radiculitis) after the administration of Qalsody have been reported. You should be aware of the symptoms of these conditions while you are being treated with this medicine. See serious side effectsin section 4 of this leaflet.
A small number of cases of patients who have developed swelling of the optic nerve of the eye (papilledema) or an increase in pressure around the brain (increased intracranial pressure) have been reported in patients treated with Qalsody. See serious side effectsin section 4 of this leaflet.
Tests before treatment
You may have a urine test(to check your kidneys) and a blood test(to check that your blood is clotting properly) before starting treatment. This is because other medicines in the same group as Qalsody may affect the kidneys and the blood cells that help with clotting. You may not need to have these tests every time you are given Qalsody.
Children and adolescents
This medicine must not be given to children and adolescents under 18 years of age. The use of this medicine in patients under 18 years of age has not been studied.
Other medicines and Qalsody
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before you are given this medicine.
Pregnancy
Qalsody is not recommended during pregnancy, or in women of childbearing potential who are not using contraception.
Breastfeeding
Your doctor will help you decide whether to continue breastfeeding or start treatment with Qalsody. Your doctor will consider the potential benefits of treatment for you compared to the benefits of breastfeeding for your baby.
Driving and using machines
This medicine may affect your ability to drive or use machines. Do not drive or use machines if you notice a change in your vision with Qalsody.
Qalsody contains sodium
This medicine contains 52 mg of sodium (the main component of cooking/table salt) in each 15 ml. This is equivalent to 3% of the maximum recommended daily intake of sodium for an adult.
Qalsody contains potassium
This medicine contains less than 1 mmol (39 mg) of potassium per 15 ml dose; this is essentially “potassium-free”.
3. How Qalsody is given
The recommended dose is 100 mg of tofersen. The first three doses are given at intervals of 14 days between them on day 1, day 15, and day 29 of treatment. After that, Qalsody will be given every 28 days.
This medicine is given by injection into the spinal fluid (in the space surrounding the spinal cord) in the lower back through a lumbar puncture. This will be done by a doctor with experience in lumbar punctures.
How long Qalsody is used
Your doctor will tell you how long you should receive Qalsody. Do not stop treatment with Qalsody without talking to your doctor.
If you miss a dose of Qalsody
If you miss a dose of Qalsody, talk to your doctor so that it can be given as soon as possible.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects related to the lumbar puncture may occur during or after the administration of Qalsody. These side effects may include headache, back pain, and infection.
Serious side effects
The most serious side effects seen in patients treated with Qalsody are inflammation of the spinal cord (myelitis) or irritation and injury of the nerve roots (radiculitis). Common symptoms may include:
- weakness
- numbness
- abnormal sensations (tingling)
- pain.
Swelling of the nerve that connects the eyes to the brain (papilledema) and increased pressure around the brain (increased intracranial pressure) have also been reported. Papilledema may be a result of increased intracranial pressure. Common symptoms may include:
- blurred vision
- double vision
- loss of vision
- headache.
Cases of inflammation of the tissue that covers the brain and spinal cord (aseptic or chemical meningitis) have been reported. This is not due to an infection. Common symptoms may include:
- headache
- fever
- stiff neck
- nausea
- vomiting.
Tell your doctor immediatelyif you get any of the symptoms mentioned above.
Other side effects
Very common(may affect more than 1 in 10 people)
- pain (back pain, pain in the arms and legs)
- feeling tired
- muscle and joint pain
- fever
- increased protein or white blood cell count in the fluid that surrounds the brain and spinal cord.
Common(may affect up to 1 in 10 people)
- muscle stiffness
- nerve pain, including burning, stabbing, or tingling sensations.
Tell your doctorif you get these symptoms or any other new symptoms that worry you.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in the European Medicines Agency website. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Qalsody
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of the month shown.
Do not use this medicine if you notice particles in the solution or if the liquid in the vial is not clear and colorless.
Store in a refrigerator(between 2°C and 8°C). Do not freeze.
Keep the vial in the outer carton to protect it from light.
The Qalsody vial can be stored at room temperature (below 30°C) for a maximum of 14 days.
Unopened Qalsody vials can be removed from and returned to the refrigerator if necessary. Unopened vials can be out of the carton for a maximum of 6 hours at room temperature for a maximum of 6 days.
6. Contents of the pack and other information
What Qalsody contains
- The active substance is tofersen.
- Each 15 ml vial contains 100 mg of tofersen.
- Each ml contains 6.7 mg of tofersen.
- The other ingredients are disodium phosphate, potassium chloride, calcium chloride dihydrate, magnesium chloride hexahydrate, sodium chloride, sodium dihydrogen phosphate dihydrate, water for injections.
Appearance and pack contents
Qalsody is a clear and colorless to slightly yellowish solution for injection.
Each pack of Qalsody contains one vial.
Marketing authorisation holder and manufacturer
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Netherlands
For further information about this medicine, contact the local representative of the marketing authorisation holder:
Belgium Biogen Belgium NV/SA Tel: +32 2 2191218 | Lithuania Biogen Lithuania UAB Tel: +370 5 259 6176 |
| Luxembourg Biogen Belgium NV/SA Tel: +32 2 2191218 |
Czech Republic Biogen (Czech Republic) s.r.o. Tel: +420 255 706 200 | Hungary Biogen Hungary Kft. Tel: +36 1 899 9883 |
Denmark Biogen (Denmark) A/S Tel: +45 77 41 57 57 | Malta Pharma.MT Ltd. Tel: +356 21337008 |
Germany Biogen GmbH Tel: +49 (0)89 99 6170 | Netherlands Biogen Netherlands B.V. Tel: +31 20 542 2000 |
Estonia Biogen Estonia OÜ Tel: +372 618 9551 | Norway Biogen Norway AS Tel: +47 23 40 01 00 |
Greece Genesis Pharma SA Tel: +30 210 8771500 | Austria Biogen Austria GmbH Tel: +43 1 484 46 13 |
Spain Biogen Spain, S.L. Tel: +34 91 310 7110 | Poland Biogen Poland Sp. z o.o. Tel: +48 22 351 51 00 |
France Biogen France SAS Tel: +33 (0)1 41 37 95 95 | Portugal Biogen Portugal Sociedade Farmacêutica, Unipessoal, Lda. Tel: +351 21 318 8450 |
Croatia Biogen Pharma d.o.o. Tel: +385 (0)1 775 73 22 | Romania Ewopharma Romania SRL Tel: +40 (0)21 260 13 44 |
Ireland Biogen Idec (Ireland) Ltd. Tel: +353 (0)1 463 7799 | Slovenia Biogen Pharma d.o.o. Tel: +386 1 511 02 90 |
Iceland Icepharma hf Tel: +354 540 8000 | Slovakia Biogen Slovakia s.r.o. Tel: +421 2 323 340 08 |
Italy Biogen Italia s.r.l. Tel: +39 02 5849901 | Finland Biogen Finland Oy Tel: +358 207 401 200 |
Cyprus Genesis Pharma Cyprus Ltd Tel: +357 22765715 | Sweden Biogen Sweden AB Tel: +46 8 594 113 60 |
Latvia Biogen Latvia SIA Tel: +371 68 688 158 |
This medicine has been authorized under exceptional circumstances. This means that due to the rarity of the disease, it has not been possible to obtain complete information on this medicine. The European Medicines Agency will review any new information that may become available every year and this leaflet will be updated as necessary.
Date of last revision of this leaflet: 05/2024
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to QALSODY 100 mg INJECTABLE SOLUTIONDosage form: INJECTABLE, 25 mgActive substance: vutrisiranManufacturer: Alnylam Netherlands B.V.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 300 mg/mlActive substance: sodium oxybateManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 50 mgActive substance: riluzoleManufacturer: Zambon S.P.A.Prescription required
Online doctors for QALSODY 100 mg INJECTABLE SOLUTION
Discuss questions about QALSODY 100 mg INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions














