AMVUTTRA 25 mg Injectable Solution in Pre-filled Syringe

How to use AMVUTTRA 25 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Amvuttra 25mg solution for injection in pre-filled syringe
vutrisiran
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Amvuttra and what is it used for
- What you need to know before you use Amvuttra
- How to use Amvuttra
- Possible side effects
- Storage of Amvuttra
- Contents of the pack and further information
1. What is Amvuttra and what is it used for
The active substance of Amvuttra is vutrisiran.
What Amvuttra is used for
Amvuttra is used to treat a disease called “ATTR amyloidosis”. This disease can be inherited and can also be caused by aging. ATTR amyloidosis is caused by problems with a protein in the body called “transthyretin” (TTR). This protein is mainly formed in the liver and carries vitamin A and other substances through the body.
In people with this disease, small fibers of the TTR protein cluster together to form deposits called “amyloid”. Amyloid can build up around nerves, the heart, and other parts of the body, or inside them, and prevent them from working normally. This causes the symptoms of the disease.
How Amvuttra works
Amvuttra works by reducing the amount of TTR protein produced by the liver, which means there is less TTR protein in the blood that can form amyloid. This can help reduce the effects of this disease.
Amvuttra is only used in adults.
2. What you need to know before you use Amvuttra
Do not use Amvuttra
- if you have ever had a severe allergic reaction to vutrisiran or any of the other ingredients of this medicine (listed in section 6).
If you are not sure, consult your doctor, pharmacist, or nurse before using this medicine.
Warnings and precautions
Reduced levels of vitamin A in the blood and vitamin A supplements
Amvuttra reduces the amount of vitamin A in the blood.
Your doctor will ask you to take a daily vitamin A supplement. Take the recommended dose of vitamin A as advised by your doctor.
Signs of low vitamin A levels may include: vision problems, especially at night, dry eyes, or blurred vision.
- If you notice any change in your vision or any other eye problems while using Amvuttra, consult your doctor. Your doctor may refer you to an eye specialist for a check-up.
Too high or too low levels of vitamin A can harm the development of the fetus. Therefore, pregnancy should be excluded in women of childbearing age before starting treatment with Amvuttra, and they should use effective contraceptive methods (see section “Pregnancy, breastfeeding, and contraception” below).
- Vitamin A levels may remain low for more than 12 months after the last dose of Amvuttra.
- Inform your doctor if you plan to become pregnant. Your doctor will advise you to stop using Amvuttra and the vitamin A supplement. Your doctor will also ensure that your vitamin A levels have returned to normal before you try to become pregnant.
- Inform your doctor in case of an unplanned pregnancy. Your doctor will advise you to stop using Amvuttra. During the first 3 months of pregnancy, your doctor may advise you to stop taking the vitamin A supplement. During the last 6 months of pregnancy, your doctor may advise you to resume the vitamin A supplement if your vitamin A levels in the blood have not yet returned to normal, due to a higher risk of deficiency during the last 3 months of pregnancy.
Children and adolescents
Amvuttra is not recommended in children and adolescents under 18 years of age.
Other medicines and Amvuttra
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and contraception
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, consult your doctor or pharmacist before using this medicine.
Pregnancy
Do not use Amvuttra if you are pregnant.
Women of childbearing age
Amvuttra will reduce the level of vitamin A in your blood, which is important for the normal development of the fetus (see “Warnings and precautions” above).
- If you are a woman who can become pregnant, you must use an effective contraceptive method during treatment with Amvuttra.
- Consult your doctor or nurse about suitable contraceptive methods.
- Pregnancy should be excluded before starting treatment with Amvuttra.
- Inform your doctor if you plan to become pregnant or in case of an unplanned pregnancy. Your doctor will advise you to stop taking Amvuttra.
Breastfeeding
It is not known whether vutrisiran can pass into breast milk. Your doctor will weigh the potential benefits of treatment for you against the risks of breastfeeding for the baby.
Driving and using machines
Amvuttra is not expected to affect your ability to drive or use machines. Your doctor will tell you if your condition allows you to drive vehicles and use machines safely.
Amvuttra contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per ml; this is, essentially “sodium-free”.
3. How to use Amvuttra
Amvuttra can be self-administered or administered by a caregiver or healthcare professional.
Your doctor or healthcare professional will show you and/or your caregiver how to prepare and inject a dose of Amvuttra before you do it yourself.
To know how to use Amvuttra, read the “Instructions for use” at the end of this leaflet.
How much Amvuttra to use
The recommended dose is 25 mg once every 3 months.
Where to administer the injection
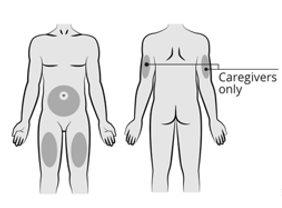
Amvuttra is administered by injection under the skin (“subcutaneous injection”) in the stomach area (abdomen), in the upper arm (if administered by another person), or in the thigh.
How long to use Amvuttra
Your doctor will tell you how long you need to use Amvuttra. Do not stop treatment with Amvuttra unless your doctor tells you to.
If you use more Amvuttra than you should
In the unlikely event that you use too much (an overdose), contact your doctor or pharmacist, even if you do not have any symptoms. Your doctor will check for any side effects.
If you forget to use Amvuttra
If you miss a dose, administer Amvuttra as soon as possible. From then on, resume administration every 3 months, counting from the last administered dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects:
Common:may affect up to 1 in 10 people
- Redness, pain, itching, bruising, or warmth at the injection site
- Blood tests showing increased levels of liver enzymes called alkaline phosphatase and alanine transaminase
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Amvuttra
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label, on the carton, and on the blister after EXP. The expiry date refers to the last day of the month stated.
Do not store above 30°C.
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Amvuttra
- The active ingredient is vutrisiran.
Each prefilled syringe contains vutrisiran sodium equivalent to 25 mg of vutrisiran in 0.5 ml of solution.
- The other components are: sodium dihydrogen phosphate dihydrate, disodium phosphate dihydrate, sodium chloride, and water for injectable preparations. Sodium hydroxide and phosphoric acid may be used to adjust the pH (see "Amvuttra contains sodium" in section 2).
Appearance and Container Contents of the Product
This medicinal product is a clear, colorless to yellowish injectable solution. Each container contains a single-dose prefilled syringe.
Marketing Authorization Holder and Manufacturer
Alnylam Netherlands B.V.
Antonio Vivaldistraat 150
1083 HP Amsterdam
Netherlands
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Alnylam Netherlands B.V. Tel: 0800 81 443 (+32 234 208 71) | Luxembourg/Luxemburg Alnylam Netherlands B.V. Tel: 80085235 (+352 203 014 48) |
Czech Republic Medison Pharma s.r.o. Tel: + 31 20 369 7861 Denmark Alnylam Sweden AB Tlf: 433 105 15 (+45 787 453 01) | Lithuania Medison Pharma Lithuania UAB Tel: +31 20 369 7861 Hungary Medison Pharma Hungary Kft Tel.: +31 20 369 7861 Malta Genesis Pharma (Cyprus) Ltd Tel: +357 22765715 |
Germany Alnylam Germany GmbH Tel: 08002569526 (+49 8920190112) | Netherlands Alnylam Netherlands B.V. Tel: 0800 282 0025 (+31 20 369 7861) |
Estonia Medison Pharma Estonia OÜ Tel: +31 20 369 7861 | Norway Alnylam Sweden AB Tlf: 800 544 00 (+472 1405 657) |
Greece ΓΕΝΕΣΙΣ ΦΑΡΜΑ Α.Ε Τηλ: +30 210 87 71 500 Spain Alnylam Pharmaceuticals Spain SL Tel: 900810212 (+34 910603753) | Austria Alnylam Austria GmbH Tel: 0800070339 (+43 720 778 072) Poland Medison Pharma Sp. z o.o. Tel: +31 20 369 7861 |
France Alnylam France SAS Tél: 0805542656 (+33 187650921) | Portugal Alnylam Portugal Tel: 707201512 (+351 21 269 853) |
Croatia Genesis Pharma Adriatic d.o.o Tel: +385 1 5530 011 | Romania Genesis Biopharma Romania SRL Tel: +40 21 403 4074 |
Ireland Alnylam Netherlands B.V. Tel: 1800 924260 (+353 818 882213) Iceland Alnylam Netherlands B.V. Sími: +31 20 369 7861 | Slovenia Genesis Biopharma SL d.o.o Tel: +386 1 292 70 90 Slovakia Medison Pharma s.r.o. Tel: +31 20 369 7861 |
Italy Alnylam Italy S.r.l. Tel: 800 90 25 37 (+39 02 89 73 22 91) | Finland Alnylam Sweden AB Puh/Tel: 0800 417 452 (+358 942 727 020) |
Cyprus Genesis Pharma (Cyprus) Ltd Τηλ: +357 22765715 | Sweden Alnylam Sweden AB Tel: 020109162 (+46 842002641) |
Latvia Medison Pharma Latvia SIA Tel: +31 20 369 7861 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu/.
-------------------------------------------------------------------------------------------------------------------
INSTRUCTIONS FOR USE
Amvuttra 25 mg solution for injection in a prefilled syringe
vutrisiran
Single-dose prefilled syringe with needle shield
Read these instructions before using this prefilled syringe.
Information about the Prefilled Syringe
The prefilled syringe (referred to as "syringe") is disposable and for single use only.
Route and Method of Administration
Each carton contains a single-dose Amvuttra syringe. Each Amvuttra syringe contains 25 mg of vutrisiran for injection under the skin (subcutaneous injection), once every 3 months.
Your doctor or healthcare professional will show you and/or your caregiver how to prepare and inject a dose of Amvuttra before you do it yourself. Contact your healthcare professional or doctor for more information and assistance if needed.
Keep these instructions until you have used the syringe.
Storage of Amvuttra
Do notstore above 30°C.
Do notfreeze.
Keep this medicinal product out of the sight and reach of children.
Important Warnings
Do notuse the medicinal product if the carton is damaged or shows signs of tampering.
Do notuse the syringe if it has been dropped onto a hard surface.
Do nottouch the plunger rod until you are ready to inject.
Do notremove the needle shield cap until just before injection.
Do notreplace the needle shield cap on the syringe at any time.
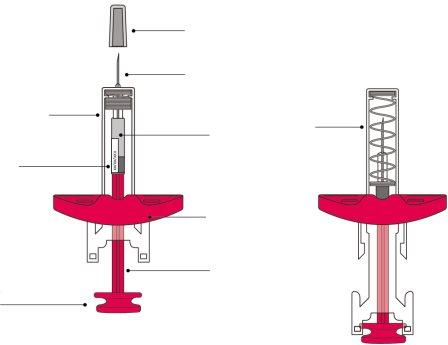
Appearance of the Syringe before and after use: |
Before useAfter use
|
Step 1: Gather Materials Gather and place the following materials (not provided) on a flat and clean surface:
|
|
Step 2: Prepare the Syringe If stored in the refrigerator, let the syringe come to room temperature for 30 minutes before use. Do notheat the syringe in any other way, e.g., microwave, hot water, or near other heat sources. Remove the syringe from the packaging by holding the syringe cylinder. Do nottouch the plunger rod until you are ready to administer the injection. Do notuse the syringe if it has been dropped onto a hard surface. Do notremove the needle shield cap until just before injection. |
|
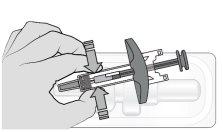
Step 3: Inspect the Syringe Check:
It is normal to see air bubbles inside the syringe. Do notuse the syringe if you detect any problems when checking the syringe and the medicinal product solution. Do notuse it if it has passed the expiration date. Do notuse it if the medicinal product solution contains particles or is cloudy or has changed color. Contact your healthcare professional if you detect any problems. |
|
Step 4: Choose the Injection Site Choose an injection site from the following areas:
Do notinject into skin areas that are sensitive, red, swollen, bruised, or hard, or within 5 cm of the navel. |
|
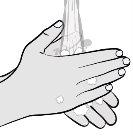
Step 5: Prepare for Injection Wash your hands with water and soap and dry them well with a clean towel. |
|
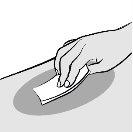
Clean the chosen injection site with an alcohol swab. Let the skin dry before injecting. Avoid touching or blowing on the injection site after cleaning. |
|
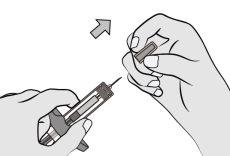
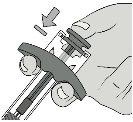
Step 6: Remove the Needle Shield CapHold the syringe cylinder with one hand. Remove the needle shield cap by pulling it straight off with the other hand and discard it immediately. It is normal to see a drop of liquid at the tip of the needle. Do nottouch the needle or let it touch any surface. Do notreplace the needle shield cap on the syringe. Do notpull the plunger rod. Do notuse the syringe if it has been dropped onto a hard surface. |
|
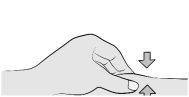
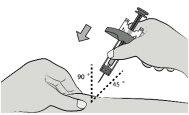
Step 7: Insert the Needle With your free hand, gently pinch the cleaned skin around the injection site to create a small bulge for injection. |
|
Insert the needle completely into the pinched skin at a 45-90° angle. |
|
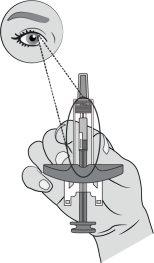
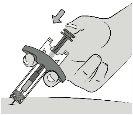
Step 8: Inject the Medicinal Product Using the push rod, press the plunger rod while holding the syringe by the wings. |
|
Press the plunger rod all the way down to inject all of the medicinal product solution. You must press the plunger rod all the way downto administer the dose. |
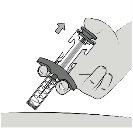
|
Step 9: Release the Plunger Rod Release the plunger rod to cover the needle. Remove the syringe from the skin. Do notblock the movement of the plunger rod. Do notpull the needle shield down. The needle shield will automatically cover the needle. |
|
Step 10: Check the Injection Site There may be a small amount of blood or liquid at the injection site. If so, press on the injection site with a gauze or cotton ball until it stops bleeding. Avoid rubbing the injection site. | |
Step 11: Dispose of the Syringe Immediately discardthe used syringe in a sharps container. Use onlya sharps container to discard syringes. |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AMVUTTRA 25 mg Injectable Solution in Pre-filled SyringeDosage form: ORAL SOLUTION/SUSPENSION, 300 mg/mlActive substance: sodium oxybateManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 50 mgActive substance: riluzoleManufacturer: Zambon S.P.A.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 10 mgActive substance: fampridineManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for AMVUTTRA 25 mg Injectable Solution in Pre-filled Syringe
Discuss questions about AMVUTTRA 25 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions