PULMICORT TURBUHALER 400 micrograms/inhalation INHALATION POWDER

How to use PULMICORT TURBUHALER 400 micrograms/inhalation INHALATION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is PULMICORT TURBUHALER 400 micrograms inhalation powder and what is it used for
- What you need to know before you use PULMICORT TURBUHALER 400 micrograms inhalation powder
- How to use PULMICORT TURBUHALER 400 micrograms inhalation powder
- Possible Adverse Effects
- Conservation of PULMICORT TURBUHALER 400 micrograms powder for inhalation
- Package Contents and Additional Information
Introduction
Package Leaflet: Information for the User
PULMICORT TURBUHALER 400 MICROGRAMS/INHALATION
INHALATION POWDER
(Budesonide)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the pack
- What Pulmicort Turbuhaler 400 micrograms inhalation powder is and what it is used for
- What you need to know before you use Pulmicort Turbuhaler 400 micrograms inhalation powder
- How to use Pulmicort Turbuhaler 400 micrograms inhalation powder
- Possible side effects
- Storage of Pulmicort Turbuhaler 400 micrograms inhalation powder
- Contents of the pack and other information
1. What is PULMICORT TURBUHALER 400 micrograms inhalation powder and what is it used for
Pulmicort Turbuhaler is presented as an inhalation powder. The powder contains only budesonide. When inhaled through the mouthpiece of the inhaler, the medicine reaches the lungs.
Budesonide belongs to a group of medicines called glucocorticoids, which are used to reduce inflammation. Pulmicort Turbuhaler reduces and prevents inflammation of the airways that causes your disease (asthma or chronic obstructive pulmonary disease, COPD).
Pulmicort Turbuhaler 400 micrograms is indicated for the maintenance treatment of diseases that involve inflammation of the airways (asthma or COPD). However, it does not relieve an acute asthma attack once it has started. It should be used regularly, as prescribed by your doctor.
2. What you need to know before you use PULMICORT TURBUHALER 400 micrograms inhalation powder
Do not use Pulmicort Turbuhaler 400 micrograms inhalation powder:
- if you are allergic (hypersensitive) to budesonide.
Warnings and precautions
- If you have previously experienced any unusual reaction to other medicines, inform your doctor.
- In certain cases, Pulmicort Turbuhaler should be administered with special caution. You should always inform your doctor of any other conditions you may have, especially if you have or have had pulmonary tuberculosis, other recent infections, or liver problems.
- This medicine has been prescribed for you to treat the disease you are suffering from (asthma or COPD). Do not use it for another condition unless your doctor tells you to. Do not give it to another person.
- Contact your doctor if you experience blurred vision or other visual disturbances.
Children
- If administered to children, your doctor will periodically review their growth, as this medicine may cause a growth delay.
Use of Pulmicort Turbuhaler 400 micrograms inhalation powder with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes medicines obtained without a prescription and herbal remedies. Some medicines may increase the effects of Pulmicort, so your doctor will monitor you closely if you are taking these medicines. In particular, inform your doctor or pharmacist if you are using any of the following medicines:
- Medicines for treating fungal infections (such as itraconazole and ketoconazole).
- Medicines for HIV (such as ritonavir or cobicistat).
- Cimetidine (a medicine for stomach acidity).
Use in athletes
Athletes are informed that this medicine contains a component that may produce a positive result in doping tests.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
There is no evidence that Pulmicort Turbuhaler 400 micrograms inhalation powder can harm the mother or child during pregnancy or breastfeeding. However, you should contact your doctor as soon as possible if you become pregnant during treatment with Pulmicort Turbuhaler. Pulmicort passes into breast milk, but in minimal amounts that do not affect the infant.
Use in children
Pulmicort should always be administered under adult supervision to ensure correct administration of the medicine.
Driving and using machines
Pulmicort does not affect your ability to drive or use tools or machines.
3. How to use PULMICORT TURBUHALER 400 micrograms inhalation powder
Follow exactly the instructions for administration of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Method of use and route of administration
This medicine is administered by inhalation.
Your doctor will indicate the duration of your treatment with Pulmicort Turbuhaler. Do not exceed the recommended treatment duration.
Before starting to use Pulmicort Turbuhaler for the first time, it is important that you read the information included in the section “How to use Pulmicort Turbuhaler 400 micrograms inhalation powder” and follow the instructions carefully.
Children may not use the dosing system correctly. Therefore, it is essential to ensure that the child can follow the instructions for use correctly.
It is recommended that the dose of Pulmicort Turbuhaler be gradually reduced when stopping use of this medicine, do not stop using it abruptly.
Remember to rinse your mouth with water after each inhalation to eliminate the part of the medicine that remains in the mouth without being absorbed by the lungs and could produce side effects.
Dosage, frequency of administration, and duration of treatment
The dose of Pulmicort Turbuhaler must be individualized. Your doctor will indicate the duration of your treatment with Pulmicort Turbuhaler. Do not administer more doses than your doctor has indicated.
Each dose of Pulmicort Turbuhaler 400 micrograms inhalation powder contains 400 micrograms of budesonide.You may require another dose of Pulmicort Turbuhaler, (see section 6. “Other presentations”).
The recommended doses of Pulmicort Turbuhaler are as follows:
Asthma
Normal dose
- For adults and elderly:100-1,600 micrograms per day, divided into 1-4 administrations.
If your doctor has prescribed low doses (100-400 micrograms per day), the daily dose can be administered in one single occasion (in the morning or at night).
- For children 6 years or older:100-800 micrograms per day, divided into 1-4 administrations.
If your doctor has prescribed low doses (100-400 micrograms per day), the daily dose can be administered in one single occasion (in the morning or at night).
In cases of severe asthma and during exacerbation of the disease, some patients may achieve improvement by dividing the daily dose into 3-4 administrations per day.
You may start to feel better from the first day of using Pulmicort Turbuhaler, although it may take 1 or 2 weeks to achieve a full effect. Therefore, it is essential that you do not stop using Pulmicort Turbuhaler even when you feel well.
If your doctor has prescribed Pulmicort Turbuhaler and you are still under treatment with corticosteroid tablets for asthma, your doctor may gradually reduce the dose of tablets (over a period of weeks or months) until you finally stop the previous treatment.
In the case of changing treatment from corticosteroid tablets to Pulmicort Turbuhaler, some symptoms of your disease may temporarily reappear, such as weakness and pain in the muscles and joints. If any of these symptoms concern you, or you experience other symptoms such as headache, fatigue, nausea, or vomiting, contact your doctor.
COPD
The recommended dose is 400 micrograms twice a day. Follow your doctor's instructions carefully, which may differ from the information contained in this leaflet.
If your doctor has prescribed Pulmicort Turbuhaler and you are still under treatment with oral corticosteroids, your doctor may gradually reduce the dose of tablets (over a period of weeks or months) and may even finally stop the previous treatment.
In the case of changing treatment from oral corticosteroids to Pulmicort Turbuhaler, some symptoms you previously experienced may temporarily reappear, such as weakness or pain in the muscles and joints. If any of these symptoms concern you, or you experience other symptoms such as headache, fatigue, nausea, or vomiting, contact your doctor.
Instructions for use/handling
Please read the instructions carefully before you start using your medicine.
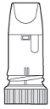
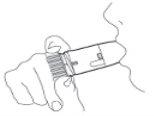
Pulmicort Turbuhaler is a multi-dose inhaler that administers small doses of powder. (Figure 1) When you inhale through the Turbuhaler, the powder will be released to your lungs. Therefore, it is essential that you breathe in strongly and deeplythrough the mouthpiece. |
Figure 1 |
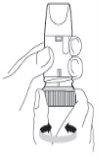
Preparing the Turbuhaler for the first time: Before using Pulmicort Turbuhaler for the first time, you must prepare the inhaler for use. Unscrew and lift the cap. Hold the inhaler in a vertical position with the thread at the bottom (Figure 2). Do not hold the mouthpiece when turning the thread. Turn the thread until it stops in one direction and then turn it until it stops in the other direction.It does not matter which direction you turn first. During this procedure, you will hear a click. You must perform this procedure twice. |
Figure 2 |
In this moment, the inhaler is ready to be used and you should not repeat the previous procedure. To inhale a dose, please follow the instructions indicated below. |
HOW TO USE PULMICORT TURBUHALER To administer a dose, simply follow the following instructions. | |
| |
| |
|
Figure 3 |
| |
| |
| |
|
NOTE:
Never exhale through the mouthpiece.
Always replace the cap properly after use.
Since the amount of powder dispensed is very small, you may not notice any taste after inhalation. However, be sure that you have inhaled the dose if you have followed the instructions.
Cleaning
The mouthpiece should be cleaned regularly (1 time a week) with a dry cloth. Do not use water to clean the mouthpiece.
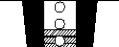
Dose indicator
When you first see a red mark in the indicator window, there are approximately 20 doses left (Figure 4). When the red mark reaches the bottom edge of the window, the inhaler will not deliver the correct amount of medicine and should be discarded (Figure 5). The sound you hear when shaking the inhaler is due to an anti-static agent and not the medicine.


Disposal
Always ensure that you dispose of your used Turbuhaler responsibly, as some medicine will remain inside.

If you use more PULMICORT TURBUHALER 400 micrograms inhalation powder than you should
If you have used more Pulmicort Turbuhaler than you should, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used. It is recommended that you take the package and the leaflet of the medicine to the healthcare professional.
It is essential that you use the dose indicated on the packaging (space reserved for the pharmacist) or the dose prescribed by your doctor, as using more inhalations will increase the risk of side effects without improving efficacy. Do not increase or decrease your dose without consulting your doctor.
If you forget to use PULMICORT TURBUHALER 400 micrograms inhalation powder
If you forget to use any of the doses of Pulmicort Turbuhaler, perform the inhalations as soon as you remember and then continue with your usual treatment. Do not use a double dose to make up for forgotten doses.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Adverse effects are usually not produced during the use of Pulmicort Turbuhaler. However, inform your doctor of the following adverse effects that bother you or do not disappear:
Frequent Adverse Effects: May affect up to 1 in 10 people
- Mild throat irritation.
- Cough.
- Hoarseness.
- Fungal infection of the mouth and throat.
- Pneumonia (lung infection) in patients with COPD.
Inform your doctor if you have any of the following symptoms while inhaling budesonide, as they may be symptoms of a lung infection:
- Fever or chills
- Increased production of mucus, change in the color of the mucus
- Increased cough or increased difficulty breathing
Uncommon Adverse Effects: May affect up to 1 in 100 people
- Cataracts (loss of transparency of the lens in the eye).
- Anxiety.
- Depression.
- Tremors.
- Muscle cramps.
- Blurred vision.
Rare Adverse Effects: May affect up to 1 in 1000 people
- Allergic reactions, including skin rash, contact dermatitis, urticaria, and angioedema (inflammation of the face, lips, and/or tongue with difficulty swallowing and breathing).
- Bruises on the skin.
- Behavioral changes (especially in children).
- Restlessness.
- Nervousness.
- As with other inhaled treatments, bronchospasm (i.e., contraction of the airways, which causes "wheezing") can rarely occur.
- Effects on the adrenal glands (small glands located next to the kidneys).
- Growth retardation.
Adverse Effects of Unknown Frequency that may include
- Sleep disorders, hyperactivity, or aggressiveness.
- Glaucoma (increased eye pressure).
Inhaled corticosteroids may affect the normal production of steroid hormones in the body, especially if high doses are used for a long time. These effects include:
- Changes in bone mineral density (decrease in bone mass).
These effects are much less likely with inhaled corticosteroids than with corticosteroid tablets.
In rare cases, other general effects of inhaled corticosteroid treatment may occur, which may be suspected if you feel tired, or have a headache, nausea, or vomiting.
In some children and adolescents treated with Pulmicort, a small decrease in growth (of approximately 1 cm) has been observed, which usually occurs only during the first year of treatment, and the corresponding adult height is finally recovered.
If you consider that any of the adverse effects you suffer from is serious or if you notice any adverse effect not mentioned in this prospectus, inform your doctor or pharmacist.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is an adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of PULMICORT TURBUHALER 400 micrograms powder for inhalation
Keep this medicine out of the sight and reach of children.
Always put the cap back on after using Pulmicort Turbuhaler. Do not store at a temperature above 30 °C.
Do not use this medicine after the expiration date that appears on the packaging and on the label of the inhaler after CAD/EXP. The expiration date is the last day of the month indicated.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Pulmicort Turbuhaler 400 micrograms powder for inhalation
The active ingredient is budesonide, each inhaled dose contains 400 micrograms. It does not contain propellants or any other type of components (excipients).
Appearance of the Product and Package Contents
Each package contains a dosing inhaler that can provide 100 doses.
Other Presentations
- PULMICORT TURBUHALER 100 MICROGRAMS/INHALATION POWDER FOR INHALATION
- PULMICORT TURBUHALER 200 MICROGRAMS/INHALATION POWDER FOR INHALATION
Marketing Authorization Holder and Manufacturer
Holder:
AstraZeneca Farmacéutica Spain S.A.
C/ Puerto de Somport 21-23
28050 Madrid
Spain
Manufacturer:
AstraZeneca AB
S-151 36 Södertälje
Sweden
Date of the Last Revision of this Prospectus: November 2017
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price24.52 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PULMICORT TURBUHALER 400 micrograms/inhalation INHALATION POWDERDosage form: PULMONARY INHALATION, 0.25 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.04000 gActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for PULMICORT TURBUHALER 400 micrograms/inhalation INHALATION POWDER
Discuss questions about PULMICORT TURBUHALER 400 micrograms/inhalation INHALATION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions