PULMICORT TURBUHALER 100 micrograms/inhalation INHALATION POWDER

How to use PULMICORT TURBUHALER 100 micrograms/inhalation INHALATION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is PULMICORT TURBUHALER 100 micrograms inhalation powder and what is it used for
- What you need to know before you use PULMICORT TURBUHALER 100 micrograms inhalation powder
- How to use PULMICORT TURBUHALER 100 micrograms inhalation powder
- Possible side effects
- Storage of PULMICORT TURBUHALER 100 micrograms inhalation powder
- Package contents and additional information
Introduction
Package Leaflet: Information for the User
PULMICORT TURBUHALER 100 MICROGRAMS/INHALATION
INHALATION POWDER
(Budesonide)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the pack
- What Pulmicort Turbuhaler 100 micrograms inhalation powder is and what it is used for
- What you need to know before you use Pulmicort Turbuhaler 100 micrograms inhalation powder
- How to use Pulmicort Turbuhaler 100 micrograms inhalation powder
- Possible side effects
- Storing Pulmicort Turbuhaler 100 micrograms inhalation powder
- Contents of the pack and other information
1. What is PULMICORT TURBUHALER 100 micrograms inhalation powder and what is it used for
Pulmicort Turbuhaler is presented as an inhalation powder. The powder contains only budesonide. When inhaled through the mouthpiece of the inhaler, the medicine reaches the lungs.
Budesonide belongs to a group of medicines called glucocorticoids, which are used to reduce inflammation. Pulmicort Turbuhaler reduces and prevents inflammation of the airways that causes asthma.
Pulmicort Turbuhaler 100 micrograms is indicated for the maintenance treatment of asthma. However, it does not relieve an acute asthma attack once it has started. It should be used regularly, as prescribed by your doctor.
2. What you need to know before you use PULMICORT TURBUHALER 100 micrograms inhalation powder
Do not use Pulmicort Turbuhaler 100 micrograms inhalation powder:
- if you are allergic (hypersensitive) to budesonide.
Warnings and precautions
- If you have previously experienced any unusual reaction to other medicines, inform your doctor.
- In certain cases, Pulmicort Turbuhaler should be administered with special caution. You should always inform your doctor of any other conditions you may have, especially if you have or have had pulmonary tuberculosis, other recent infections, or liver problems.
- This medicine has been prescribed for you to treat the disease you are suffering from (asthma). Do not use it for another condition unless your doctor tells you to. Do not give it to another person.
- Contact your doctor if you experience blurred vision or other visual disturbances.
Children
- If administered to children, your doctor will periodically review their growth since this medicine may cause a delay in growth.
Using Pulmicort Turbuhaler 100 micrograms inhalation powder with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes medicines obtained without a prescription and herbal remedies. Some medicines may increase the effects of Pulmicort, so your doctor will monitor you closely if you are taking these medicines. In particular, inform your doctor or pharmacist if you are using any of the following medicines:
- Medicines for treating fungal infections (such as itraconazole and ketoconazole).
- Medicines for HIV (such as ritonavir or cobicistat).
- Cimetidine (a medicine for stomach acidity).
Use in athletes
Athletes are informed that this medicine contains a component that may produce a positive result in doping tests.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
No evidence suggests that Pulmicort Turbuhaler 100 micrograms inhalation powder can harm the mother or child during pregnancy or breastfeeding. However, you should contact your doctor as soon as possible if you become pregnant during treatment with Pulmicort Turbuhaler. Pulmicort passes into breast milk, but in minimal amounts that do not affect the infant.
Use in children
Pulmicort should always be administered under adult supervision to ensure correct administration of the medicine.
Driving and using machines
Pulmicort does not affect your ability to drive or use tools or machines.
3. How to use PULMICORT TURBUHALER 100 micrograms inhalation powder
Follow exactly the instructions for administration of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Form of use and route of administration
This medicine is administered by inhalation.
Your doctor will indicate the duration of your treatment with Pulmicort Turbuhaler. Do not exceed the recommended treatment duration.
Before starting to use Pulmicort Turbuhaler for the first time, it is essential that you read the information included in the section “How to use Pulmicort Turbuhaler 100 micrograms inhalation powder” and follow the instructions carefully.
Children may not use the dosing system correctly. Therefore, it is crucial to ensure that the child can follow the instructions for use correctly.
It is recommended that the dose of Pulmicort Turbuhaler be gradually reduced when stopping use of this medicine, do not stop using it abruptly.
Remember to rinse your mouth with water after each inhalation to eliminate the part of the medicine that remains in the mouth without being absorbed by the lungs, which could produce side effects.
Dosage, frequency of administration, and duration of treatment
The dose of Pulmicort Turbuhaler must be individualized. Your doctor will indicate the duration of your treatment with Pulmicort Turbuhaler. Do not administer more doses than your doctor has indicated.
Each dose of Pulmicort Turbuhaler 100 micrograms inhalation powder contains 100 micrograms of budesonide. You may require another dose of Pulmicort Turbuhaler (see section 6, “Other presentations”).
The recommended doses of Pulmicort Turbuhaler are as follows:
Normal dose
- For adults and the elderly:100-1,600 micrograms per day, divided into 1-4 administrations.
If your doctor has prescribed low doses (100-400 micrograms per day), the daily total dose can be administered in one occasion (in the morning or at night).
- For children 6 years of age or older:100-800 micrograms per day, divided into 1-4 administrations.
If your doctor has prescribed low doses (100-400 micrograms per day), the daily total dose can be administered in one occasion (in the morning or at night).
You may start to feel better from the first day of using Pulmicort Turbuhaler, although it may take 1 or 2 weeks to achieve a full effect. Therefore, it is essential that you do not stop using Pulmicort Turbuhaler even when you feel better.
If your doctor has prescribed Pulmicort Turbuhaler and you are still under treatment with corticosteroid tablets for asthma, your doctor may gradually reduce the dose of tablets (over a period of weeks or months) until you finally stop the previous treatment.
In the event that you have changed treatment from corticosteroid tablets to Pulmicort Turbuhaler, some symptoms of your disease may temporarily reappear, such as weakness and pain in the muscles and joints. If any of these symptoms concern you, or if you experience any other symptoms such as headache, fatigue, nausea, or vomiting, contact your doctor.
Instructions for use/handling
Please read the instructions carefully before starting to use your medicine.
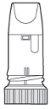
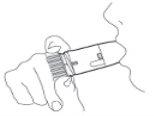
Pulmicort Turbuhaler is a multidose inhaler that administers small doses of powder. (Figure 1) When you inhale through the Turbuhaler, the powder will be released to your lungs. Therefore, it is essential that you breathe in energetically and deeplythrough the mouthpiece. |
Figure 1 |
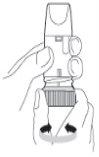
Preparing the Turbuhaler for the first time: Before using Pulmicort Turbuhaler for the first time, you must prepare the inhaler for use. Unscrew and lift the cap. Hold the inhaler in a vertical position with the thread in the lower part (Figure 2). Do not hold the mouthpiece when turning the thread. Turn the thread to the stop in one direction and then turn it back to the stop in the other direction.It does not matter which direction you turn first. During this procedure, you will hear a click. You must perform this procedure twice. |
Figure 2 |
At this moment, the inhaler is ready to be used, and you should not repeat the above procedure. To inhale a dose, please follow the instructions indicated below. |
HOW TO USE PULMICORT TURBUHALER To administer a dose, simply follow the following instructions. | |
| |
| |
|
Figure 3 |
| |
| |
| |
|
NOTE:
Never exhale through the mouthpiece.
Always replace the cap properly after use.
Since the amount of powder dispensed is very small, you may not notice any taste after inhalation. However, be sure that you have inhaled the dose if you have followed the instructions.
Cleaning
The mouthpiece should be cleaned regularly (1 time a week) with a dry cloth. Do not use water to clean the mouthpiece.
Dose indicator
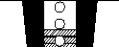
When you first see a red mark in the indicator window, there are approximately 20 doses left (Figure 4). When the red mark reaches the lower edge of the window, the inhaler will not deliver the correct amount of medicine and should be discarded (Figure 5). The sound you hear when shaking the inhaler is due to a desiccant and not the medicine.


Disposal
Make sure to always dispose of your used Turbuhaler responsibly, as some medicine will remain inside.

If you use more PULMICORT TURBUHALER 100 micrograms inhalation powder than you should
If you have used more Pulmicort Turbuhaler than you should, consult your doctor, pharmacist, or call the Toxicological Information Service, telephone 91 562 04 20, indicating the medicine and the amount used. It is recommended to take the package and the package leaflet of the medicine to the healthcare professional.
It is essential that you use the dose indicated on the packaging (space reserved for the pharmacist) or the one your doctor has prescribed, as using more inhalations will increase the risk of side effects without improving efficacy. Do not increase or decrease your dose without consulting your doctor.
If you forget to use PULMICORT TURBUHALER 100 micrograms inhalation powder
If you forget to use any of the doses of Pulmicort Turbuhaler, take the recommended inhalations as soon as you remember and then continue with your usual treatment. Do not use a double dose to make up for forgotten doses.
If you have any other questions about using this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Usually, no side effects occur during the use of Pulmicort Turbuhaler. However, inform your doctor of the following side effects that bother you or do not disappear:
Common side effects: May affect up to 1 in 10 people
- Mild throat irritation.
- Cough.
- Hoarseness.
- Fungal infection of the mouth and throat.
- Pneumonia (lung infection) in patients with COPD.
Tell your doctor if you experience any of the following symptoms while inhaling budesonide; they could be symptoms of a lung infection:
- Fever or chills
- Increased production of mucus, change in the color of mucus
- Increased cough or increased difficulty breathing
Uncommon side effects: May affect up to 1 in 100 people
- Cataracts (loss of transparency of the lens in the eye).
- Anxiety.
- Depression
- Tremors.
- Muscle cramps.
- Blurred vision.
Rare side effects: May affect up to 1 in 1,000 people
- Allergic reactions, including skin rash, contact dermatitis, urticaria, and angioedema (inflammation of the face, lips, and/or tongue with difficulty swallowing and breathing).
- Bruises on the skin.
- Behavioral changes (especially in children).
- Restlessness.
- Nervousness.
- As with other inhaled treatments, bronchospasm (i.e., contraction of the airways, which causes wheezing) can rarely occur.
- Effects on the adrenal glands (small glands located next to the kidneys).
- Growth delay.
Side effects of unknown frequency that may include:
- Sleep disorders, hyperactivity, or aggressiveness.
- Glaucoma (increased eye pressure).
Corticosteroids inhaled may affect the normal production of steroid hormones in the body, especially if high doses are used for a long time. These effects include:
- changes in bone mineral density (decrease in bone mass).
These effects are much less likely with inhaled corticosteroids than with corticosteroid tablets.
In rare cases, other general effects of inhaled corticosteroid treatment may occur, which may be suspected if you feel tired, or experience headache, nausea, or vomiting.
In some children and adolescents treated with Pulmicort, a small decrease in growth (of approximately 1 cm) has been observed, which usually occurs only during the first year of treatment, finally recovering the corresponding adult height.
If you consider that any of the side effects you are experiencing is severe or if you notice any side effect not mentioned in this leaflet, inform your doctor or pharmacist.
Reporting of side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of PULMICORT TURBUHALER 100 micrograms inhalation powder
Keep this medicine out of the sight and reach of children.
Always put the cap back on after using Pulmicort Turbuhaler. Do not store above 30 ºC.
Do not use this medicine after the expiry date which is stated on the packaging and on the inhaler label after CAD/EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package contents and additional information
Composition of Pulmicort Turbuhaler 100 micrograms inhalation powder
The active ingredient is budesonide, each inhaled dose contains 100 micrograms of budesonide. It does not contain propellants or any other type of components (excipients).
Appearance of the product and package contents
Each package contains a dosing inhaler that can provide 200 doses.
Other presentations
- PULMICORT TURBUHALER 200 MICROGRAMS/INHALATION INHALATION POWDER
- PULMICORT TURBUHALER 400 MICROGRAMS/INHALATION INHALATION POWDER
Marketing authorization holder and manufacturer
Holder:
AstraZeneca Farmacéutica Spain S.A.
C/ Puerto de Somport 21-23
28050 Madrid
Spain
Manufacturer:
AstraZeneca AB
S-151 36 Södertälje
Sweden
Date of last revision of this leaflet: November 2017
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price12.25 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PULMICORT TURBUHALER 100 micrograms/inhalation INHALATION POWDERDosage form: PULMONARY INHALATION, 0.25 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.04000 gActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for PULMICORT TURBUHALER 100 micrograms/inhalation INHALATION POWDER
Discuss questions about PULMICORT TURBUHALER 100 micrograms/inhalation INHALATION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions