Ondemet
Ask a doctor about a prescription for Ondemet

How to use Ondemet
Leaflet accompanying the packaging: patient information
Ondemet, 0.25 mg/mL, nebulizer suspension
Ondemet, 0.5 mg/mL, nebulizer suspension
Budesonide
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Ondemet and what is it used for
- 2. Important information before taking Ondemet
- 3. How to take Ondemet
- 4. Possible side effects
- 5. How to store Ondemet
- 6. Contents of the packaging and other information
1. What is Ondemet and what is it used for
Ondemet belongs to a group of medicines called glucocorticosteroids (cortisone). Medicines in this group have anti-inflammatory effects.
Ondemet is indicated for:
- treatment, reduction, and prevention of inflammatory conditions of the respiratory tract in asthma
- treatment of symptoms of chronic obstructive pulmonary disease by reducing inflammation of the respiratory tract
- treatment of very severe croup syndrome (laryngitis that can cause breathing difficulties)
Ondemet should not be used as a substitute for a bronchodilator medicine.
2. Important information before taking Ondemet
When not to take Ondemet
- if the patient is allergic to budesonide or any of the other ingredients of the medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Ondemet, discuss it with your doctor or pharmacist if:
- the patient has or has had liver disease or liver problems
- they have pulmonary tuberculosis (active or inactive)
- they have a fungal or viral infection of the respiratory tract
In the case of switching from a corticosteroid medicine to Ondemet, in some cases, allergic symptoms may recur, such as rhinitis and eczema. The patient may also feel tired, have headaches, muscle and joint pain, and sometimes nausea and vomiting. This is because the amount of cortisone produced by the body decreases with long-term use of a corticosteroid medicine. These problems usually go away after some time of continuing treatment with Ondemet, but if the symptoms are severe, you should immediatelycontact your doctor.
After taking the medicine, rinse your mouth with water to minimize the risk of oral thrush and pharyngitis. You should contact your doctor if symptoms of a fungal infection occur.
In rare cases, during long-term treatment with Ondemet, the growth of children and adolescents may be slowed down. If a child takes this medicine for a long time, the doctor will usually regularly check the child's growth.
In the event of asthma worsening, consult a doctor. This may mean that the dosage needs to be changed or other treatment is needed.
In the event of an acute asthma attack, use a fast-acting bronchodilator.
If you experience blurred vision or other vision disturbances, contact your doctor.
Children and adolescents
The doctor should regularly check the growth of children who are taking Ondemet for a long time.
If growth is slowed down, the therapy should be re-evaluated.
Ondemet and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take, including non-prescription and herbal medicines.
Some medicines may affect treatment with Ondemet, such as those containing:
- ketokonazole or itraconazole (found in medicines used for fungal infections).
- saquinavir, indinavir, ritonavir, nelfinavir, amprenavir, lopinavir, fosamprenavir, atazanavir, or tipranavir (so-called HIV protease inhibitors used in HIV treatment).
Ondemet may affect the result of a pituitary function test, the ACTH stimulation test, giving falsely low values.
Pregnancy and breastfeeding
Experience with use during pregnancy does not indicate an increased risk of developmental abnormalities. However, before taking the medicine during pregnancy, consult a doctor, as the severity of asthma may change and treatment may need to be modified.
Budesonide passes into breast milk. The effect of therapeutic doses of Ondemet on breastfed infants is considered unlikely. Ondemet can be used during breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before taking this medicine.
Driving and using machines
Ondemet does not affect the ability to drive or use machines.
3. How to take Ondemet
Always take this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
Your doctor will tell you how much medicine to take. This will depend on the severity of your disease.
Ondemet should be taken daily or as directed by your doctor, even if you do not have symptoms of asthma.
Ondemet is given using a nebulizer (inhalation device). During inhalation through the mouthpiece or face mask, the medicine is delivered with the inhaled air to the respiratory tract. Therefore, it is essential to breathe evenly and calmly during administration – see the nebulizer instruction manual.
Nebulizer instruction manual.
Ondemet should only be used with a special inhalation device called a nebulizer.
Instructions for using Ondemet ampoules
- 1. Separate the required number of ampoules from the strip. Leave the remaining ampoules in the sachet to protect them from light.
- 2. Shake the ampoule(s) gently for 30 seconds.
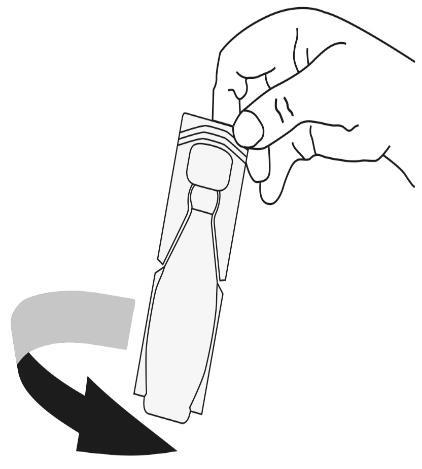
- 3. Hold the ampoule upright. Unscrew the top part of the ampoule(s).
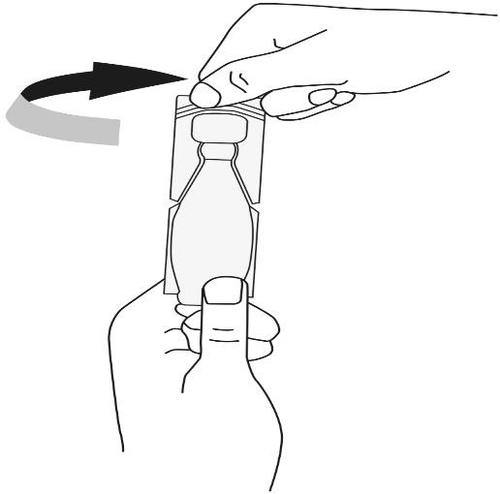
- 4. Pour the prescribed amount of medicine into the nebulizer chamber. The single-dose container is marked with a line. If only 1 ml is to be used, hold the single-dose container upside down and empty it until the liquid level reaches the line.
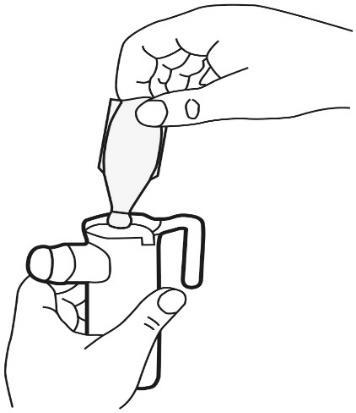
- 5. Use the nebulizer according to the manufacturer's instructions and your doctor's recommendations.
- 6. Dispose of used ampoules. If the ampoule contains unused contents, it can be used within 12 hours of opening the ampoule.
- 7. Using a face mask or mouthpiece, breathe in the mist from the nebulizer calmly and deeply, sitting or standing upright. When using a face mask, make sure it fits well. For children, a face mask can be used to make it easier for them to inhale.
- 8. Rinse your mouth with water. Spit out the water. Do not swallow. When using a face mask, also wash your face.
- 9. After each use, wash the medicine reservoir and mouthpiece of the nebulizer (or face mask) with mild detergent, then rinse thoroughly and dry. Refer to the nebulizer cleaning and disinfection instructions.
An opened single-dose ampoule should be used within 12 hours and stored away from light before use.
Since the nebulizer chamber should always contain at least 2 ml at the start of nebulization, if the patient is to inhale only 1 ml of Ondemet, it should be diluted with saline (sodium chloride 9 mg/ml [0.9%] for injection).
Taking a higher dose of Ondemet than recommended
If too much Ondemet has been taken at one time, it is unlikely to cause harm.
If higher doses than recommended by the doctor have been taken for a longer period (several months), there is a possibility of side effects.
In the event of taking too much medicine or, for example, accidental ingestion by a child, consult a doctor or hospital to assess the risk and get advice.
It is essential to take the dose as stated on the packaging or as directed by the doctor. Do not increase or decrease the dose without consulting your doctor.
Missing a dose of Ondemet
If a dose of Ondemet prescribed by the doctor has been missed, take the next dose. Do not take a double dose to make up for the missed dose.
In case of any further doubts about taking this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Ondemet can cause side effects, although not everybody gets them.
Severe side effects:
Common (may affect up to 1 in 10 people)
- Pneumonia (in patients with COPD).
Tell your doctor if you experience any of the following symptoms while taking Ondemet, as they may be signs of pneumonia:
- fever or chills
- increased mucus production, change in mucus color
- worsening cough or increased breathing difficulties.
Rare (may affect up to 1 in 1,000 people)
- Angioedema (swelling around the eyes, lips, genitals, hands, or feet, or other parts of the body), anaphylaxis (swelling of the lips and tongue, pressure in the throat, difficulty breathing, feeling of fainting), bronchospasm (constriction of the airway muscles).
Stop taking Ondemet and contact your doctor immediately if you experience any of the following symptoms, which may be related to angioedema, anaphylaxis, or bronchospasm:
- swelling of the face, tongue, or throat
- difficulty swallowing
- difficulty breathing
- hives
Other possible side effects:
Common (affecting less than 1 in 10 people)
- Irritation of the throat, cough, oral thrush (fungal infection of the mouth and/or throat). Oral thrush is less likely if the mouth is rinsed with water after taking Ondemet.
Uncommon (affecting less than 1 in 100 people)
- Blurred vision, cataract (clouding of the lens of the eye), depression, anxiety, muscle cramps, tremors
Rare (affecting less than 1 in 1,000 people)
- Immediate and delayed allergic reactions, such as hives and other skin rashes
- Corticosteroid side effects. Inhaled corticosteroids may affect the body's natural production of steroid hormones, especially with long-term use of high doses. Side effects include:
- Changes in bone mineral density (thinning of the bones).
- Glaucoma (increased pressure in the eye).
- Reduced growth rate in children and adolescents.
- Suppression of adrenal function (a small gland located near the kidney).
The likelihood of these side effects is much lower with inhaled corticosteroids than with oral corticosteroids.
- Bruising, dysphonia (speech disorders), hoarseness, anxiety, behavioral disturbances (mainly in children).
Side effects of unknown frequency; frequency cannot be estimated from available data
- Sleep disturbances,
- aggression, feeling of strong excitement and/or irritability.
- glaucoma (increased pressure in the eye)
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
Jerozolimskie Avenue 181C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ondemet
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the outer packaging, sachet, and ampoule after EXP. The expiry date refers to the last day of the month.
Do not freeze.
Store in the original packaging to protect from light.
Shelf life after opening the aluminum sachet: 3 months.
Shelf life after opening the ampoule: 12 hours. Note that if only 1 ml is used, the remaining volume is not sterile.
Shelf life after dilution of the medicinal product: the prepared suspension should be used within 30 minutes.
This product does not require special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Ondemet contains
- The active substance is budesonide. Ondemet 0.25 mg/mL: Each 2 mL ampoule contains 0.5 mg of budesonide. Ondemet 0.5 mg/mL: Each 2 mL ampoule contains 1 mg of budesonide.
- The other ingredients are: disodium edetate (E385), sodium chloride, polysorbate 80 (E433), anhydrous citric acid (E330), sodium citrate (E331), hydrochloric acid, and sodium hydroxide to adjust the pH, and water for injection.
What Ondemet looks like and contents of the pack
- Each ampoule contains 2 mL of a liquid with a color from white to almost white.
- The ampoules are packed in strips of 5 in a closed sachet made of aluminum foil (PET/Alu/PE).
- Package sizes: each package contains 10, 20, 40, 60, 80, and 120 ampoules. Not all package sizes may be marketed.
Marketing authorization holder
Zentiva k.s.
Dolní Měcholupy
U kabelovny 130
102 37 Prague 10
Czech Republic
Manufacturer
Genetic S.p.A.
Contrada Canfora
84084 Fisciano, Italy
This medicine is authorized in the Member States of the European Economic Area under the following names:
Sweden, Norway: Budesonide Zentiva
Poland: Ondemet
For more information, contact the marketing authorization holder's representative:
Zentiva Polska Sp. z o.o.
Bonifraterska Street 17
00-203 Warsaw
Tel.: +48 22 375 92 00
Date of last revision of the leaflet:April 2025
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGenetic S.p.A
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OndemetDosage form: Suspension, 0.125 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.25 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.5 mg/mlActive substance: budesonidePrescription required
Alternatives to Ondemet in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ondemet in Ukraine
Alternative to Ondemet in Spain
Online doctors for Ondemet
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ondemet – subject to medical assessment and local rules.