
PROMIXIN 1 Million International Units (IU), Powder for Solution for Nebulizer Inhalation


How to use PROMIXIN 1 Million International Units (IU), Powder for Solution for Nebulizer Inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
PROMIXIN
1 million International Units (IU)
powder for solution
for inhalation by nebulizer
Colistimethate sodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Promixin and what is it used for
- What you need to know before you use Promixin
- How to use Promixin
- Possible side effects
- Storage of Promixin
- Package contents and further information
1. What is Promixin and what is it used for
Promixin contains the active substance colistimethate sodium and is administered by inhalation to treat chronic respiratory infections in patients with cystic fibrosis. Promixin is used when these infections are caused by a specific bacterium called Pseudomonas aeruginosa.
This is a very common bacterium that infects the lungs of almost all patients with cystic fibrosis at some point in their lives. If the infection is not properly controlled, it will continue to damage the lungs, causing more problems.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or the common cold.
It is important that you follow the instructions regarding dosage, administration, and duration of treatment as indicated by your doctor.
Do not store or reuse this medicine. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not throw medicines down the drain or in the trash.
To administer Promixin, the powder in the vial must be dissolved with a suitable solvent, sterile saline solution, or sterile water, and then inhaled (inhaled) into the lungs using a suitable inhalation device, so that most of the antibiotic can reach the bacteria that cause the infection.
2. What you need to know before you use Promixin
In certain circumstances, your doctor may decide not to prescribe Promixin.
Do not use Promixin:
- If you are allergic(hypersensitive) to colistimethate sodium, colistin, or other polymyxins;
If you are in this situation, inform your doctor before starting to take Promixin.
Warnings and precautions with Promixin and inform your doctor:
- If you have or have had kidney problems;
- If you suffer from myasthenia gravis(a rare disease in which your muscles are extremely weak and you get tired quickly);
- If you suffer from porphyria(a rare metabolic disease with which some people are born);
- If you suffer from asthma.
If you are in any of these situations, inform your doctor.
In premature infants and newborns, caution should be exercised when using Promixin because the kidneys are not yet fully developed.
Other medicines and Promixin:
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. These medicines may interfere with the effects of Promixin.
- medicines that may affect how your kidneys work. Taking these medicines at the same time as Promixin may increase the risk of kidney damage.
- medicines that may affect the nervous system. Taking these medicines at the same time as Promixin may increase the risk of side effects on your nervous system.
- medicines called muscle relaxants, often used during general anesthesia. Promixin may increase the effects of these medicines. If you are going to be given a general anesthetic, inform your anesthetist that you are using Promixin.
If you have myasthenia gravis and are also taking other antibiotics called macrolides (such as azithromycin, clarithromycin, or erythromycin), or antibiotics called fluoroquinolones (such as ofloxacin, norfloxacin, and ciprofloxacin), taking Promixin increases the risk of muscle weakness and respiratory difficulties even more.
Receiving colistimethate sodium by infusion at the same time as receiving Promixin by inhalation may increase your risk of side effects.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, if you think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
There is no information on the safety of Promixin in pregnant women. Your doctor should advise you before using Promixin when the benefits of the medicine outweigh the risk.
Colistimethate sodium may be secreted in breast milk. Please discuss the use of Promixin with your doctor.
Driving and using machines
Promixin may cause dizziness, confusion, or vision problems, such as blurred vision. If this happens, do not drive or use tools or machines.
3. How to use Promixin
Follow your doctor's instructions for administering this medicine exactly. If in doubt, consult your doctor or pharmacist again.
Consult your doctor if you have kidney problems, as you may need a lower dose of Promixin.
You should use your first doseof Promixin in the presence of your doctor or nurse.
Use Promixin after physiotherapy(if you have physiotherapy). This will ensure that your lungs are clear so that Promixin can work effectively. If you are also taking other inhaled medicines, your doctor will tell you in what order to take them.
The recommended dose in adults, adolescents, and children 2 years of age or older is 1-2 vials(1-2 million units) two or three times a day(up to 6 million units per day).
The recommended dose in children under 2 years of age is half to 1 vial (0.5-1 million units) twice a day (up to 2 million units per day).
Your doctor may decide to adjust the dose depending on your circumstances.
Promixin is inhaled from a device called a nebulizer. Promixin can be administered using any nebulization system that can be used for the release of antibiotics into the lungs in the form of mist. In countries where the I-neb AAD system is available, Promixin is presented with a Promixin Disk that can be used with the I-neb AAD system. For information on using Promixin with the I-neb system, see the detailed instructions provided with the device. If you use a different nebulizer, make sure the room you are in is well ventilated.
How to prepare Promixin
Your doctor or nurse will show you how to prepare and use Promixin with the nebulizer.
Before putting Promixin in the nebulizer and inhaling it, you must first dissolve it with sterile water, sterile saline solution 0.9%or a mixture of equal parts of sterile water and sterile saline solution 0.9%as indicated below. Your doctor or nurse will indicate the correct volume of liquid to be added to each vial of Promixin and how many vials of Promixin to use for each dose with your nebulizer.
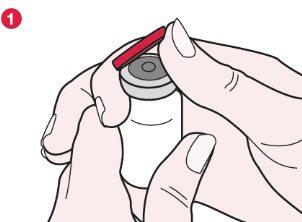
- Locate the edge on the red plastic cap near the arrow with the mark “FLIP UP”. Then, holding the vial with one hand and the plastic cap with the other, turn the cap slightly counterclockwise. Put your thumb under the edge and push the cap up about 90° (see diagram 1).
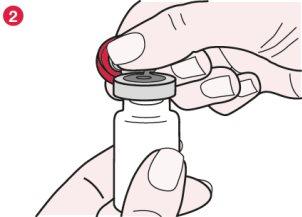
- Take the plastic cap as shown in diagram 2 and pull it slowly, like a hinge, until almost 180°.
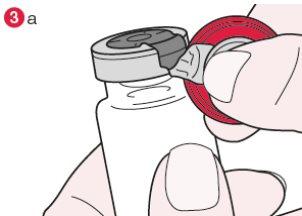
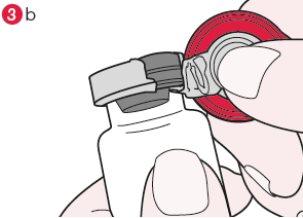
- Turn the vial so that the plastic cap is facing you. Holding the cap by the center as shown, pull it down and turn it slightly to the right (diagram 3a) or to the left so that the metal seal breaks only on one side (diagram 3b).
- Once the seal is broken, hold the vial firmly and remove the metal seal to expose the entire rubber cap (diagram 4).
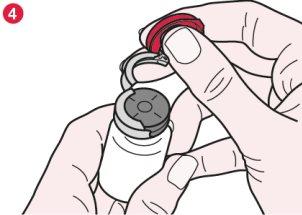
- Remove the rubber cap from the Promixin vial by holding only the outer edge of the cap and place it upside down on a clean surface. Slowly add sterile water, sterile saline solution 0.9%, or a mixture of equal parts of sterile water and sterile saline solution 0.9% to the vial, until the volume of liquid indicated by your doctor or nurse is reached.
- Put the rubber cap back on and gently turn the vial upside down twice (diagram 6).
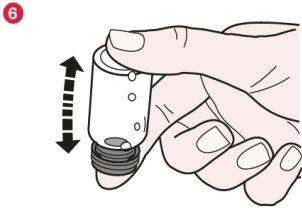
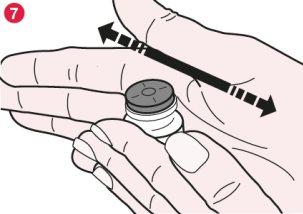
- Gently roll the vial between your hands to dissolve all visible Promixin powder at the bottom and side of the vial (diagram 7). Do not shake the vial too hard as this could cause the solution to foam.
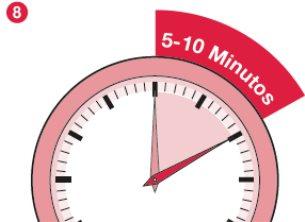
- When most of the powder has dissolved, let the vial stand for 5-10 minutes to allow any foam to disappear and for any remaining powder to dissolve.
Pour the solution into the nebulizer and inhale immediately. If it is not possible to use the solution immediately, put the vial back on and store it in the refrigerator for a maximum of 24 hours. Do not use any solution that has been prepared for more than 24 hours.See section 5 “Storage of Promixin” for instructions on how to store or dispose of any unused Promixin.
If you use more Promixin than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
The symptoms of using too much Promixin may include:
- tingling or numbness around the lips and face
- dizziness and feeling of vertigo
- poorly articulated speech
- visual disturbance
- confusion
- mental alteration
- flushing (redness of the face)
If you forget to use Promixin
Apply your dose as soon as you remember, unless it is close to the time of your next dose. Do not take a double dose to make up for the forgotten dose.
If you stop using Promixin
Do not stop your treatment early unless your doctor tells you to.
Your doctor will tell you the duration of your treatment.
If you have more questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Promixin may sometimes cause allergic reactionssuch as skin rashes. If this happens, you should stop using Promixinand tell your doctor immediately.
Inhaling Promixin through a nebulizer may cause some people to experience chest tightness, wheezing, coughing, or a feeling of shortness of breath (sometimes described as a feeling of choking). For this reason, the first dose should be administered in the presence of your doctor or nurse. Your doctor may also advise you to take a medicine to prevent breathing difficulties. Your doctor should check your breathing during visits.
Promixin could also affect the kidneys, usually if the dose is high or if you are taking other medicines that may affect the kidneys.
Promixin may occasionally cause pain in the mouth or throat.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Promixin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date that appears on the vial and on the carton after EXP. The expiry date is the last day of the month indicated.
Unopened Promixin vials do not require special storage conditions.
Promixin does not contain preservatives. Once prepared, Promixin solutions should be used preferably immediately. If this is not possible, the solutions should not be stored for more than 24 hours in a refrigerator. Do not use any solution that has been prepared for more than 24 hours.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package contents and further information
Promixin composition
The active substance is colistimethate sodium.
Each vial contains 1 million International Units (IU) of colistimethate sodium, which weighs approximately 80 milligrams (mg). It does not contain other ingredients.
Appearance of the product and package contents
Promixin is a powder for solution for inhalation by nebulizer, supplied as a white or off-white powder in a glass vial.
Promixin is supplied in packs containing 30 vials. In countries where the I-neb AAD system is available, each pack also contains a Promixin Disk for use with the I-neb AAD system.
Marketing authorization holder
Zambon S.p.A.
Via Lillo del Duca 10
20091 Bresso (MI) – Italy
Phone: +39 02 665241
e-mail: [email protected]
Manufacturer
Xellia Pharmaceuticals ApS
Dalslandsgade 11,
DK- 2300, Copenhagen S, Denmark.
You can request more information about this medicine by contacting the local representative of the marketing authorization holder
Zambon S.A.U.
Maresme 5. Pol. Can Bernades-Subirà
08130 Sta. Perpètua de Mogoda – Barcelona
Phone: 93 544 64 00
Fax: 93 574 04 36
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria, Netherlands, Sweden, France: Tadim
Germany, Denmark, Norway, United Kingdom (Northern Ireland), Italy, Spain, Portugal: Promixin
Date of last revision of this leaflet: June 2022
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PROMIXIN 1 Million International Units (IU), Powder for Solution for Nebulizer InhalationDosage form: PULMONARY INHALATION, 1 MIUActive substance: colistinManufacturer: Pari Pharma GmbhPrescription requiredDosage form: PULMONARY INHALATION, 2 MIUActive substance: colistinManufacturer: Pari Pharma GmbhPrescription requiredDosage form: INJECTABLE, 1 IUActive substance: colistinManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for PROMIXIN 1 Million International Units (IU), Powder for Solution for Nebulizer Inhalation
Discuss questions about PROMIXIN 1 Million International Units (IU), Powder for Solution for Nebulizer Inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















