COLFINAIR 1 Million IU Powder for Solution for Nebulizer Inhalation


How to use COLFINAIR 1 Million IU Powder for Solution for Nebulizer Inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Colfinair 1 millionUIpowder for solution for inhalation by nebulizer
sodium colistimethate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Colfinair and what is it used for
- What you need to know before you use Colfinair
- How to use Colfinair
- Possible side effects
- Storage of Colfinair
- Contents of the pack and other information
1. What is Colfinair and what is it used for
Antibiotics are used to treat bacterial infections and are not effective against viral infections. It is essential that you follow the instructions regarding dosage, administration interval, and treatment duration as indicated by your doctor. Do not store or reuse this medication. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not throw away medications via the drain or in the trash. |
Colfinair is administered by inhalation to treat chronic respiratory infections in patients with cystic fibrosis. Colfinair is used when such infections are caused by a specific bacterium called Pseudomonas aeruginosa.
2. What you need to know before you use Colfinair
Do not use Colfinair
- If you are allergic (hypersensitive) to sodium colistimethate, colistin, or other polymyxins.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Colfinair
- If you have or have had kidney problems
- If you have myasthenia gravis
- If you have porphyria
- If you have asthma
In premature infants and newborns, caution should be exercised when using Colfinair because the kidneys are not yet fully developed.
Coughing and chest tightness may lead to interruption. This can be alleviated with the use of an inhaled bronchodilator (e.g., salbutamol) before using Colfinair. Your doctor will supervise your first dose of Colfinair and check your lung function before and after administration.
If, despite using a bronchodilator, you experience chest tightness, inform your doctor because this may indicate an allergic reaction and treatment should be discontinued.
During treatment with Colfinair, neurotoxicity may occur with possible dizziness, confusion, or visual disturbances. If you experience side effects such as dizziness, confusion, or visual disturbances, or any not mentioned in this leaflet, inform your doctor.
Taking Colfinair with other medicines
- medicines that may affect how your kidneys work. Taking such medicines at the same time as Colfinair may increase the risk of kidney damage.
- medicines that may affect the nervous system. Taking such medicines at the same time as Colfinair may increase the risk of side effects on your nervous system.
- medicines called muscle relaxants, often used during general anesthesia. Colfinair may increase the effects of these medicines. If you are going to be administered general anesthesia, inform your anesthesiologist that you are using Colfinair.
If you have myasthenia gravis and are also taking other antibiotics called macrolides (such as azithromycin, clarithromycin, or erythromycin), or antibiotics called fluoroquinolones (such as ofloxacin, norfloxacin, and ciprofloxacin), taking Colfinair increases the risk of muscle weakness and respiratory difficulties even further.
Receiving sodium colistimethate by infusion at the same time as receiving Colfinair by inhalation may increase your risk of experiencing side effects.
Colfinair must not be mixed with any other medicine in the nebulizer!
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Colfinair has a moderate influence on the ability to drive and use machines. During treatment with Colfinair, neurotoxicity may occur with possible dizziness, confusion, or visual disturbances. If you experience any side effects such as dizziness, confusion, or visual disturbances, do not drive or use machines and consult your doctor or pharmacist.
3. How to use Colfinair
Colfinair is for inhalation use only.
Follow exactly the administration instructions of this medicine as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is:
Proposed dose | Maximum dose per day | |
Adults, Adolescents(12 to 17 years),Children(2 to 11 years) | 1-2 million units, two or three times a day | 6 million units |
Children under2 years | 0.5-1 million units, twice a day | 2 million units |
Your doctor may decide to adjust the dose depending on your circumstances. If you are also taking other inhaled medications, your doctor will indicate the order in which you should take them.
Note that Colfinair is also available in a 2 million UI vial, which may be a more suitable dose depending on the prescribed dosage by the doctor.
For use in children under 2 years of age, the PARI LC SPRINT Baby (red nozzle adapter) with a mask is recommended.
Your doctor will tell you how long your treatment with Colfinair will last. Do not stop treatment prematurely because when treating bacterial infections, it is essential to complete the full course of treatment to reduce the risk of developing resistance to the infectious bacterium.
Preparation for inhalation treatment
If you are being treated at home, your doctor or nurse will show you how to use Colfinair in your nebulizer when you start this treatment.
To start your treatment, you will need the following:
|
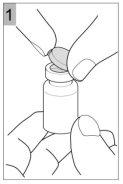
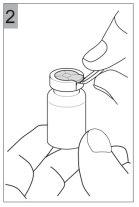
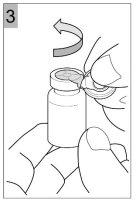
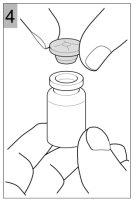
How to prepare Colfinair for inhalation
Before you can introduce Colfinair into the nebulizer and inhale it, it must be dissolved with 3 ml of sterile solution for inhalation of NaCl 0.9%. An ampoule contains the correct volume to dissolve Colfinair.
Colfinair must be used immediately after dissolution. Do not dissolve Colfinair until you are ready to administer a dose (see also section 5)
|
| |
|
| |
|
| |
|
|
Step 5
- Add 3 ml of sterile solution for inhalation of NaCl 0.9% to Colfinair 1 millionUI(red cap)
- Replace the rubber stopper and gently turn the vial twice.
- Roll the vial gently between your hands to dissolve all visible Colfinair powder at the bottom and side of the vial. Do not shake the vial too hard, as this may cause the solution to foam.
- Once most of the powder has dissolved, let the vial sit for the foam to disappear and the remaining powder to dissolve. This may take a few minutes.
Use Colfinair only when all the powder has dissolved and there are no visible particles in the solution.
How to use Colfinair
Colfinair is for inhalation use only with a suitable nebulizer (e.g., eFlowrapidor PARI LC SPRINT).
Read the nebulizer's instructions for use carefully to obtain more information about handling the nebulization system.
It is essential that your nebulization system functions correctly before starting treatment with Colfinair.
Place the components of your nebulizer on a flat, clean surface and follow the manufacturer's instructions for use.
Inhalation should be done in a well-ventilated room.
After inhaling Colfinair
Consult the manufacturer's instructions for use of the nebulizer for cleaning and disinfecting the same.
If you use more Colfinair than you should
If you have used more Colfinair than you should, consult your doctor or pharmacist immediately. If too much Colfinair is administered accidentally, the effects can be severe and may include kidney problems, muscle weakness, and respiratory difficulties (even arrest).
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone 915 620 420 (indicating the medication and the amount ingested), or go to the nearest hospital.
If you forget to use Colfinair
If you are treating yourself and have forgotten a dose, you should administer the forgotten dose as soon as you remember and then the next dose 8 or 12 hours later, and continue from then on as indicated.
Do not take a double dose to make up for forgotten doses.
If you stop treatment with Colfinair
You must not stop using Colfinair as prescribed without consulting your doctor first.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
An allergic reaction may occur. Severe allergic reactions can occur even with the first dose and may include rapid onset of rash, swelling of the face, tongue, and throat, inability to breathe due to narrowing of the airways, and loss of consciousness. Urgent medical attention will be required.
If you think you have an allergic reaction to Colfinair, inform your doctor immediately.
Some side effects can be serious
Very common: may affect more than 1 in 10 people.
- Chest tightness due to narrowing of the airways (may not always be a true allergic reaction)
Frequency not known: cannot be estimated from the available data.
- Patients with severe kidney impairment and at higher doses may experience known side effects of intravenous administration
- Confusion
- Psychotic disorder
- Visual disturbance
- Dizziness
If you experience any of these effects, inform your doctor immediately.
Other possible side effects
Very common: may affect more than 1 in 10 people.
- Sores in the mouth or throat
- Cough
- Difficulty breathing
- Wheezing
- Worsening of lung function test results
- Transient absence of spontaneous breathing
If any of these problems affect you severely, inform your doctor.
Frequency not known: cannot be estimated from the available data.
- Subjective skin sensations
- Speech disorder
- Dizziness
- Kidney failure
Other possible side effects include oral or pharyngeal candidiasis.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Colfinair
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton or label after EXP. The expiry date is the last day of the month indicated.
Do not store above 25°C.
Keep the vial in the outer carton to protect it from light.
Colfinair solution for inhalation by nebulizer should be used immediately after preparation. If this is not possible, a Colfinair solution should be stored in the refrigerator (2°C - 8°C) and not more than 24 hours.
If not used immediately, storage conditions and times are the responsibility of the user.
Any leftover solution should be discarded.
For single use only.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Colfinair
- The active ingredient is sodium colistimethate.
- Each 10 ml vial contains 1 million IU, which is equivalent to approximately 80 mg of sodium colistimethate.
Appearance of the Product and Container Contents
Colfinair is a powder for solution for inhalation by nebulizer.
1 million IU/vial: | White powder in a 10 ml colorless glass vial with a red cap. |
Also available: 2 million IU/vial: | White powder in a 10 ml colorless glass vial with a lavender cap. |
The product is available in the following package sizes:
Cardboard box containing:
- 8 cardboard boxes of 7 vials each (56 vials),
- 2 cardboard boxes of 0.9% NaCl inhalation solution with 30 ampoules of 3 ml each (60 ampoules), and a handheld nebulizer device eFlowrapid.
Marketing Authorization Holder and Manufacturer
PARI Pharma GmbH
Moosstrasse 3
82319 Starnberg
Germany
Tel.: +49 (0) 89/74 28 46 – 10
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
PARI Pharma Iberia S.L.
Miguel Yuste 17, 4th floor C.
28037 Madrid
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
GermanyColiFin 1 Million. I.E. Powder for solution for inhalation by nebulizer
NetherlandsColiFin PARI 1,000,000 IE Powder for nebulizer solution
AustriaColiFin 1 Mio. I.E. Powder for solution for inhalation by nebulizer
SpainColfinair 1 million IU powder for solution for inhalation by nebulizer
ItalyColfinair 1,000,000 U Powder for nebulizer solution
Date of the last revision of this leaflet: 05/2022
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLFINAIR 1 Million IU Powder for Solution for Nebulizer InhalationDosage form: PULMONARY INHALATION, 2 MIUActive substance: colistinManufacturer: Pari Pharma GmbhPrescription requiredDosage form: INJECTABLE, 1 IUActive substance: colistinManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 2 IUActive substance: colistinManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for COLFINAIR 1 Million IU Powder for Solution for Nebulizer Inhalation
Discuss questions about COLFINAIR 1 Million IU Powder for Solution for Nebulizer Inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions