POTENCIATOR 5 g ORAL SOLUTION

Ask a doctor about a prescription for POTENCIATOR 5 g ORAL SOLUTION

How to use POTENCIATOR 5 g ORAL SOLUTION
Introduction
Leaflet: information for the user
Potenciator 5 g oral solution
Arginine aspartate
Read the entire leaflet carefully before starting to take this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 15 days.
Contents of the leaflet
- What Potenciator oral solution is and what it is used for
- What you need to know before taking Potenciator oral solution
- How to take Potenciator oral solution
- Possible side effects
- Storage of Potenciator oral solution
- Package contents and additional information
1. What Potenciator oral solution is and what it is used for
Potenciator 5g oral solution contains arginine aspartate as the active ingredient. Arginine is an amino acid (a component of proteins) that is involved in many processes in the human body.
It is indicated for the prevention and treatment of amino acid deficiency states when not enough protein is taken in the diet, due to unbalanced or restrictive diets (for weight loss, vegetarian); convalescence.
Potenciator 5g oral solution is indicated for adults and adolescents over 12 years of age.
2. What you need to know before taking Potenciator oral solution
Do not take Potenciator
- If you are allergic to arginine aspartate or any of the other components of this medication (listed in section 6).
- If you are pregnant.
Due to the high dose it contains, do not take it:
- If you are breastfeeding.
- Children under 12 years of age.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Potenciator oral solution.
- Do not exceed the recommended doses and do not take this medication continuously for long periods of time.
- If you have any liver or kidney disease, or diabetes, you should consult your doctor before taking this medication.
- In patients with cystic fibrosis, digestive cramps, weight loss, and abdominal swelling may occur.
Children and adolescents
This medication is contraindicated in children under 12 years of age.
Taking Potenciator with other medications
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
The active ingredient of this medication interacts with the following medications:
- Potassium-sparing diuretics (used for arterial hypertension) such as amiloride, spironolactone, or triamterene.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Do not take Potenciator oral solution during pregnancy.
Breastfeeding women should not take this medication.
Driving and using machines
No effects have been observed that affect these abilities.
Potenciator oral solution contains sucrose, sorbitol (E-420), methyl parahydroxybenzoate (E-218), and propyl parahydroxybenzoate (E-216)
This medication contains sucrose and sorbitol (E-420). If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
It may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate (E-218) and propyl parahydroxybenzoate (E-216).
3. How to take Potenciator oral solution
Follow the administration instructions for the medication contained in this leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
Adults and adolescents (from 12 years old):1 drinkable ampoule per day, during meals.
Treatment should not generally exceed 2 weeks.
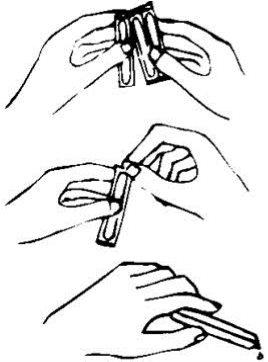
To correctly administer this medication, follow the instructions indicated below:
| 1.- Separate the ampoule 2.- Tear the tab at the top with a rotary motion 3.- After inverting it, squeeze the ampoule and pour the contents into a glass |
The contents of the ampoule can be taken alone or with water or fruit juice.
Use in children
Do not administer to children under 12 years of age.
If you take more Potenciator oral solution than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or go to a medical center, or call the Toxicology Information Service Telephone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take Potenciator oral solution
Do not take a double dose to make up for forgotten doses.
If you have any other doubts about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone experiences them.
The following side effects have been described, whose frequency cannot be estimated from the available data:
Some cases of thrombocytopenia (decrease in platelet count) and hematuria (presence of blood in the urine) have been reported.
In rare cases, allergic reactions (hypersensitivity) to one of the components of the medication may occur.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: www.notificaRAM.es
By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Potenciator oral solution
No special storage conditions are required.
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the packaging after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown down the drain or into the trash. Deposit the packaging and medications you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Potenciator 5 g oral solution
- The active ingredient is arginine aspartate. Each 10 ml ampoule contains 5 g of arginine aspartate.
- The other components (excipients) are: sorbitol (E-420) (70% solution), sucrose, citric acid monohydrate, sodium saccharin, caramel flavor, caramel color, potassium sorbate (E-202), methyl parahydroxybenzoate (E-218), propyl parahydroxybenzoate (E-216), and deionized water.
Appearance of the product and package contents
Potenciator is a brown-colored oral solution with a caramel odor, presented in plastic ampoules.
Each package contains 20 ampoules.
Marketing authorization holder
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
Spain
Manufacturer
Faes Farma, S.A.
Maximo Agirre Kalea, 14
48940 Leioa (Bizkaia)
Spain
O
Faes Farma, S.A.
Parque Científico y Tecnológico de Bizkaia
Ibaizabal Bidea, Edificio 901
48160 Derio (Bizkaia)
Spain
Date of the last revision of this leaflet: July 2014.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to POTENCIATOR 5 g ORAL SOLUTIONDosage form: TABLET, 200 mgActive substance: carglumic acidManufacturer: Laboratorios Tillomed Spain S.L.UPrescription requiredDosage form: TABLET, 200 mgActive substance: carglumic acidManufacturer: Waymade B.V.Prescription required
Alternatives to POTENCIATOR 5 g ORAL SOLUTION in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to POTENCIATOR 5 g ORAL SOLUTION in Ukraine
Online doctors for POTENCIATOR 5 g ORAL SOLUTION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for POTENCIATOR 5 g ORAL SOLUTION – subject to medical assessment and local rules.