PLYZARI 6 mg/mL injectable solution in prefilled pen


How to use PLYZARI 6 mg/mL injectable solution in prefilled pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Plyzari 6 mg/ml Solution for Injection in Pre-filled Pen
Liraglutide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Plyzari and what is it used for
- What you need to know before you start using Plyzari
- How to use Plyzari
- Possible side effects
- Storage of Plyzari
- Contents of the pack and other information
1. What is Plyzari and what is it used for
What is Plyzari
Plyzari is a weight-loss medicine that contains the active substance liraglutide. It is similar to a naturally occurring hormone called glucagon-like peptide-1 (GLP-1) that is released from the intestine after eating. Liraglutide works on the brain's appetite control centers, making you feel fuller and less hungry. This can help you eat less and reduce your body weight.
What Plyzari is used for
Liraglutide is used for weight loss in combination with a suitable diet and exercise in adults over 18 years of age with:
- a BMI of 30 kg/m2 or higher (obesity) or
- a BMI of 27 kg/m2 and less than 30 kg/m2 (overweight) and weight-related health problems (such as diabetes, high blood pressure, abnormal blood fats, or breathing problems during sleep called "obstructive sleep apnea").
Body Mass Index (BMI) is a measure of body weight in relation to height.
You should only continue using this medicine if you have lost at least 5% of your initial body weight after 12 weeks of treatment with a daily dose of 3.0 mg (see section 3). Consult your doctor before continuing.
Liraglutide can be used in combination with a healthy diet and increased physical activity to control weight in adolescents from 12 years of age and older with:
- obesity (diagnosed by your doctor)
- body weight over 60 kg
You should only continue using this medicine if you have lost at least 4% of your BMI after 12 weeks of treatment with a dose of 3.0 mg/day or the maximum tolerated dose (see section 3). Consult your doctor before continuing.
Diet and Exercise
Your doctor will put you on a diet and indicate an exercise program that you should follow while being treated with liraglutide.
2. What you need to know before you start using Plyzari
Do not use Plyzari
- if you are allergic to liraglutide or any of the other ingredients of this medicine (listed in section 6).
Warnings and Precautions
Consult your doctor, pharmacist, or nurse before starting treatment with liraglutide.
The use of this medicine is not recommended if you have severe heart failure.
There is limited experience with this medicine in patients over 75 years of age. It is not recommended if you are 75 years of age or older.
There is limited experience with this medicine in patients with kidney problems. If you have kidney disease or are on dialysis, consult your doctor.
There is limited experience with this medicine in patients with liver problems. If you have liver problems, consult your doctor.
This medicine is not recommended if you have a severe stomach or intestinal problem that causes delayed stomach emptying (called gastroparesis), or if you have an inflammatory bowel disease.
If you know you are going to have surgery that requires anesthesia (a state of sleep), inform your doctor that you are taking this medicine.
People with Diabetes
If you are diabetic, do not use this medicine as a substitute for insulin.
Pancreatitis
Consult your doctor if you have or have had a pancreatic disease.
Gallbladder Inflammation and Gallstones
If you lose a lot of weight, you are at risk of developing gallstones and, as a result, gallbladder inflammation. Stop using this medicine and contact your doctor immediately if you experience severe pain in the upper abdomen, usually worse on the right side, under the ribs. The pain can radiate to the back or right shoulder. See section 4.
Thyroid Disease
Consult your doctor if you have thyroid disease, including thyroid nodules and thyroid gland enlargement.
Heart Rate
Consult your doctor if you have palpitations (you are aware of your heartbeat) or if you have a feeling of rapid heartbeat at rest during treatment with this medicine.
Fluid Loss and Dehydration
When starting treatment with this medicine, you may lose fluids or become dehydrated. This can be due to the occurrence of nausea, vomiting, and diarrhea. It is essential to avoid dehydration by drinking plenty of fluids. If you have any doubts or questions, consult your doctor, pharmacist, or nurse. See section 4.
Children and Adolescents
The safety and efficacy of liraglutide have not been studied in children under 12 years of age.
Other Medicines and Plyzari
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
In particular, tell your doctor, pharmacist, or nurse if:
- you are taking diabetes medicines called "sulfonylureas" (such as glimepiride or glibenclamide) or if you are being given insulin. Your blood sugar level may drop (hypoglycemia) if you use these medicines with liraglutide. Your doctor may adjust the dose of your diabetes medication to prevent episodes of hypoglycemia. See section 4 for warning signs of low blood sugar. If you adjust your insulin dose, your doctor may recommend checking your blood sugar level more frequently.
- you are taking warfarin or other oral medicines that reduce blood clotting (anticoagulants). More frequent blood tests may be needed to determine your blood's clotting ability.
Pregnancy and Breastfeeding
Do not use this medicine if you are pregnant, think you may be pregnant, or plan to become pregnant, as it is not known whether liraglutide can affect the fetus.
If you are using this medicine, you should avoid breastfeeding, as it is not known whether liraglutide is excreted in breast milk.
Driving and Using Machines
Liraglutide is unlikely to affect your ability to drive or use machines.
Some patients may feel dizzy when taking this medicine, mainly during the first 3 months of treatment (see section "Possible Side Effects"). If you feel dizzy, be very careful when driving or using machines. If you need more information, consult your doctor.
Plyzari contains Sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to use Plyzari
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are in doubt, consult your doctor, pharmacist, or nurse again.
Your doctor will put you on a diet and indicate an exercise program that you should follow while being treated with this medicine.
How much to inject
Adults
Treatment will start with a low dose that will be gradually increased over the first 5 weeks of treatment.
- When you start using this medicine, the initial dose is 0.6 mg once a day for at least one week.
- Your doctor will tell you to gradually increase the dose, usually by 0.6 mg per week, until you reach the recommended dose of 3.0 mg once a day.
Your doctor will tell you how much of this medicine to use each week. You will usually be told to follow the following table.
Week | Injected dose |
Week 1 | 0.6 mg once a day |
Week 2 | 1.2 mg once a day |
Week 3 | 1.8 mg once a day |
Week 4 | 2.4 mg once a day |
Week 5 and onwards | 3.0 mg once a day |
Once you have reached the recommended dose of 3.0 mg in week 5 of treatment, continue using this dose until the end of the treatment period. Do not increase the dose further.
Your doctor will evaluate your treatment periodically.
Adolescents (≥ 12 years)
For adolescents from 12 years of age and older, follow a gradual dose increase as in adults (see the table for adults above). The dose should be increased to 3.0 mg (maintenance dose) or up to the maximum tolerated dose. Daily doses above 3.0 mg are not recommended.
How and when to use Plyzari
- Before using the pen for the first time, your doctor or nurse will show you how to use it.
- You can use this medicine at any time of day, with or without food and drink.
- Use this medicine at approximately the same time every day: choose a time that suits you best.
Where to inject
Liraglutide is administered as an injection under the skin (subcutaneous injection).
- The best injection sites are the abdomen, the front of the thigh, or the upper arm.
- Do not inject into a vein or muscle.
Injection needles are not included with the pen. For example, you can use disposable needles such as BD Ultra-FineTM or NovoFine® as fine as 32 G and up to 8 mm in length.
On the other side of this leaflet, you will find detailed instructions for use.
People with Diabetes
Tell your doctor if you have diabetes. Your doctor may adjust the dose of your diabetes medication to prevent episodes of hypoglycemia.
- Do not mix liraglutide with other injectable medicines (e.g., insulins).
- Do not use liraglutide in combination with other medicines that contain GLP-1 receptor agonists (such as exenatide or lixisenatide).
If you use more Plyzari than you should
If you use more liraglutide than you should, talk to a doctor or go to a hospital immediately. Bring the medicine pack with you. You may need medical treatment. The following effects may occur:
- nausea
- vomiting
- low blood sugar (hypoglycemia). See warning signs of low blood sugar in "Common Side Effects".
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service. Phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Plyzari
- If you forget a dose and remember within 12 hours of when you normally inject the dose, inject it as soon as you remember.
- However, if more than 12 hours have passed since you should have used this medicine, skip the missed dose and inject the next dose the following day at the usual time.
- Do not use a double dose or increase the dose the next day to make up for missed doses.
If you stop treatment with Plyzari
Do not stop treatment with liraglutide without consulting your doctor.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious Side Effects
Rarely, serious allergic reactions (anaphylaxis) have been reported in patients using liraglutide. See your doctor immediately if you have symptoms such as breathing problems, swelling of the face and throat, and palpitations.
Very rarely, cases of pancreatitis (inflammation of the pancreas) have been reported in patients using liraglutide. Pancreatitis is a serious and potentially life-threatening disease.
Stop using this medicine and contact your doctor immediately if you notice any of the following serious side effects:
- Severe and persistent abdominal pain (in the stomach area) that may radiate to the back, as well as nausea and vomiting, as it may be a sign of pancreatitis.
Other Side Effects
Very Common:may affect more than 1 in 10 people
- nausea, vomiting, diarrhea, constipation, headache; usually disappear after a few days or weeks.
Common:may affect up to 1 in 10 people
- stomach and intestinal problems such as indigestion (dyspepsia), gastritis, stomach discomfort, pain in the upper stomach, heartburn, bloating, gas (flatulence), belching, and dry mouth
- feeling weak or tired
- changes in taste
- dizziness
- difficulty sleeping (insomnia). Usually occurs during the first 3 months of treatment
- gallstones
- rash
- reactions at the injection site (such as bruising, pain, irritation, itching, and rash)
- low blood sugar (hypoglycemia). Warning signs of low blood sugar may appear suddenly and include: cold sweat, cool pale skin, headache, palpitations, nausea, excessive hunger, vision problems, drowsiness, feeling weak, nervousness, anxiety, confusion, difficulty concentrating, and trembling. Your doctor will tell you how to treat low blood sugar and what to do if you notice these warning signs
- increased pancreatic enzymes, such as lipase and amylase.
Uncommon:may affect up to 1 in 100 people
- fluid loss (dehydration). This is more likely to occur at the beginning of treatment and may be due to vomiting, nausea, and diarrhea
- delayed gastric emptying
- inflamed gallbladder
- allergic reactions including skin rashes
- feeling unwell
- rapid heartbeat.
Rare:may affect up to 1 in 1,000 people
- reduced kidney function
- acute kidney failure. Symptoms may include reduced urine output, metallic taste in the mouth, and ease of bruising (bruises).
Frequency Not Known:cannot be estimated from the available data
- intestinal obstruction. A severe form of constipation with additional symptoms such as stomach pain, bloating, vomiting, etc.
Reporting of Side Effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Plyzari
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the pen label and carton after "EXP". The expiry date is the last day of the month shown.
Before first use:
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
When you start using the pen:
You can store the pen for a month if you keep it below 30 °C or in a refrigerator (between 2 °C and 8 °C). Do not freeze.
When not in use, store the pen with the cap on to protect it from light.
Do not use this medicine if you notice that the solution is not clear and colorless or almost colorless.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the SIGRE collection point at the pharmacy. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Plyzari
- The active ingredient is liraglutide. 1 ml of injectable solution contains 6 mg of liraglutide. A pre-filled pen contains 18 mg of liraglutide.
- The other components are sodium citrate dihydrate, propylene glycol, phenol, and water for injectable preparations. Additionally, hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment.
Appearance of the Product and Container Contents
Plyzari is supplied as a clear and colorless or almost colorless injectable solution in a pre-filled pen. Each pen contains 3 ml of solution and can administer doses of 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg, and 3.0 mg.
Plyzari is available in packs of 1, 3, or 5 pens. Only certain pack sizes may be marketed.
Needles are not included.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Zentiva, k.s.
U Kabelovny 130
Dolní Mecholupy
102 37 Prague 10
Czech Republic
Manufacturer
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park,
Paola PLA 3000
Malta.
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Zentiva Spain S.L.U.
Avenida de Europa, 19, Edificio 3, Planta 1.
28224 Pozuelo de Alarcón, Madrid
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria: Luntin 6 mg/ml Injektionslösung in einem Fertigpen
Sweden, Norway, Germany: Nevolat
Netherlands: Nevolat 6 mg/ml oplossing voor injectie in een voorgevulde pen
Hungary: Nevolat 6 mg/ml oldatos injekció eloretöltött injekciós tollban
Spain: Plyzari 6 mg/ml solución inyectable en pluma precargada
Poland, Czech Republic, Italy, Portugal: Plyzari
France: LIENDAX 6 mg/mL, solution injectable en stylo prérempli
Date of the last revision of this leaflet:December 2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
INSTRUCTIONS FOR USE OF PLYZARI 6 MG/ML INJECTABLE SOLUTION IN PRE-FILLED PEN
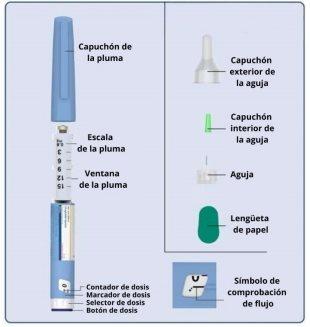
Instructions on how to use Plyzari 6 mg/ml injectable solution in pre-filled pen Read these instructions carefullybefore using the Plyzari pre-filled pen. Do not use the pen without receiving proper trainingfrom your doctor or nurse. Start by checking the pen to make sure it contains Plyzari 6 mg/mland then look at the illustrations to familiarize yourself with the different parts of the pen and the needle. If you are blind or have low vision and cannot read the dose counter on the pen, do not use this pen without help.Seek help from a person who can see well and is trained in the use of the Plyzari pre-filled pen. Your pen is a pre-filled dosing pen. It contains 18 mg of liraglutide and administers doses of 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg, and 3.0 mg. It is recommended to use disposable BD Ultra Fine TM or NovoFine® needles with this device. Needles are not included in the package. Important information Pay special attention to these notes because they are important for the safe use of the pen. | Plyzari pre-filled pen and needle (example)
|
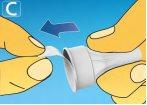
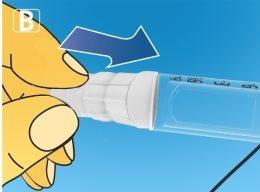
1 Preparing the pen with a new needle
|
|
|
|
|
|
|
|
|
|
A drop of solution may appear at the tip of the needle. This is normal, but you should still check the flow if you are using a new pen for the first time. Do not put a new needleon the pen until you are ready to take the injection. Always use a new needlefor each injection. This will help you avoid clogged needles, contamination, infection, and inaccurate dosing.
|
|
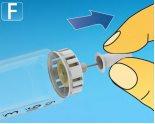
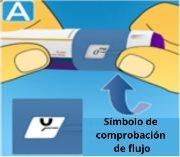
2 Checking the flow
Press and hold the dose buttonuntil the dose counter returns to 0. The 0 must be aligned with the dose marker. A drop of solution should appear at the tip of the needle. A small drop may remain at the tip of the needle, but it will not be injected. If no drop appears, repeat step 2 "Checking the flow" up to 6 times. If a drop still does not appear, change the needle and repeat step 2 "Checking the flow" one more time. If, despite this, no drop appears, discard the pen and use a new one. Always make sure a drop appearsat the tip of the needle before using a new pen for the first time. This ensures that the solution is flowing. If no drop appears, do notinject the medication, even if the dose counter moves. This may indicate that the needle is blocked or damaged. If you do not check the flow before the first injection with each new pen, you may not receive the prescribed dose, and this medication may not have the intended effect. |
|
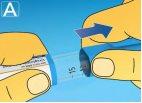
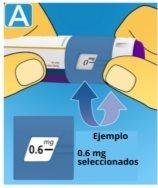
3 Selecting the dose
If you have selected an incorrect dose, you can turn the dose selector forward or backward to select the correct dose. The pen can select up to a maximum of 3.0 mg. The dose selector changes the dose. Only the dose counter and the dose marker show how many mg you have selected for each administration. You can select up to 3.0 mg per dose. When the pen contains less than 3.0 mg, the dose counter stops before 3.0 appears. The dose selector clicks differently when turned forward, backward, or when passing the number of mg remaining. Do not count the clicks of the pen. Always use the dose counter and the dose marker to see how many mg you have selected before injecting this medication. Do not count the clicks of the pen. Do not use the pen scale, as it only shows the approximate amount of solution remaining in the pen. With the dose selector, only select doses of 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg, or 3.0 mg.The selected dose must be exactly aligned with the dose marker to ensure that the injected dose is correct. |
|
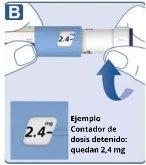
How much solution is left?
Turn the dose selector until the dose counter stops. If it shows 3.0, it means that there are at least 3.0 mgleft in the pen. If the dose counter stops before 3.0 mg, it means that there is not enough solution for a full 3.0 mg dose. If you need more medication than is left in the pen If your doctor or nurse advises you to do so and has taught you how to do it, you can divide the dose between the pen in use and a new one. Use a calculator to plan the dose as your doctor or nurse has instructed you. Be very careful to do the calculation correctly. If you are not sure how to divide the dose using two pens, select and inject the dose you need with a new pen. |
|
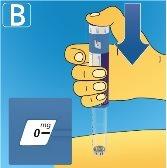
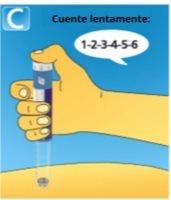
4 Injecting the dose
|
|
|
|
|
|
If blood appears at the injection site, press lightly. Do not rub the area. A drop of solution may appear at the tip of the needle after the injection. This is normal and does not affect the dose. Always check the dose counter to see how many mg you have injected.Press and hold the dose button until the dose counter shows 0. How to detect if the needle is blocked or damaged?
What to do if the needle is blocked? Change the needle as described in step 5 "After the injection" and repeat all the steps from step 1 "Preparing the pen with a new needle". Make sure to select the full dose you need. Never touch the dose counter while injecting.This can interrupt the injection. |
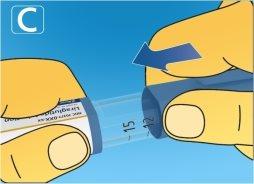
|
5 After the injection
|
|
|
|
Always discard the needle after each injectionto ensure comfortable injections and prevent clogged needles. If the needle is blocked, you will not injectany medication. When the pen is empty, discard it withoutthe needle attached, following the instructions of your doctor, nurse, pharmacist, or local authorities. Never try to put the inner needle cap back on.You could prick yourself with it. Always remove the needle from the pen after each injection. This will help you avoid clogged needles, contamination, infection, solution loss, and inaccurate dosing. |
|
More important information
| |
Care of the pen
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PLYZARI 6 mg/mL injectable solution in prefilled penDosage form: INJECTABLE, 6 mg/mlActive substance: liraglutideManufacturer: Sun Pharmaceutical Industries (Europe) B.V.Prescription requiredDosage form: INJECTABLE, 6 mg/mlActive substance: liraglutideManufacturer: Sun Pharmaceutical Industries (Europe) B.V.Prescription requiredDosage form: INJECTABLE, 6 mg/mlActive substance: liraglutideManufacturer: Zentiva K.S.Prescription required
Online doctors for PLYZARI 6 mg/mL injectable solution in prefilled pen
Discuss questions about PLYZARI 6 mg/mL injectable solution in prefilled pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions