DIAVIC 6 mg/ml INJECTABLE SOLUTION IN PRE-FILLED PEN

How to use DIAVIC 6 mg/ml INJECTABLE SOLUTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Diavic 6 mg/ml Solution for Injection in Pre-filled Pen EFG
Liraglutide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Diavic and what is it used for
- What you need to know before you start using Diavic
- How to use Diavic
- Possible side effects
- Storage of Diavic
- Contents of the pack and further information
1. What is Diavic and what is it used for
Diavic contains the active substance liraglutide. It helps your body to reduce your blood sugar level only when your blood sugar level is too high. It also slows down the movement of food from your stomach and may help to prevent heart disease.
Liraglutide is used on its own if your blood sugar level is not controlled by diet and exercise alone, and you cannot use metformin (another diabetes medicine).
Liraglutide is used together with other diabetes medicines when these are not enough to control your blood sugar level. These may include:
- oral anti-diabetics (such as metformin, pioglitazone, sulfonylurea, sodium-glucose cotransporter type 2 (SGLT2) inhibitor) and/or insulin.
2. What you need to know before you use Diavic
Do not use Diavic
- If you are allergic to liraglutide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse:
- before you start using liraglutide.
- if you have or have had pancreatitis.
If you are going to have an operation, tell your doctor that you are using Diavic.
This medicine should not be used if you have type 1 diabetes (your body does not produce any insulin) or diabetic ketoacidosis (a complication of diabetes characterized by high blood sugar levels and increased breathing rate). It is not an insulin and should not be used as a substitute for insulin.
Diavic should not be used if you are on dialysis.
Diavic should not be used if you have severe liver disease.
The use of Diavic is not recommended if you have severe heart failure.
This medicine should not be used if you have a severe stomach or intestinal problem that causes delayed emptying of the stomach (called gastroparesis) or inflammatory bowel disease.
If you get symptoms of acute pancreatitis, such as severe and persistent stomach pain, you should contact your doctor immediately (see section 4).
If you have thyroid disease, including thyroid nodules and increased thyroid gland size, talk to your doctor.
When you start using Diavic, you may experience dehydration (loss of fluids), for example, if you have vomiting, nausea, and diarrhea. It is important to avoid dehydration by drinking plenty of fluids. Talk to your doctor if you have any questions.
Children and adolescents
Diavic can be used in adolescents and children from 10 years of age. There is no data available in children under 10 years of age.
Other medicines and Diavic
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
In particular, tell your doctor, pharmacist, or nurse if you are using medicines that contain any of the following active substances:
- Sulfonylurea (such as glimepiride or glibenclamide) or insulin. You may experience hypoglycemia (low blood sugar) when using Diavic with a sulfonylurea or insulin, as sulfonylureas and insulin increase the risk of hypoglycemia. When you start using these medicines together for the first time, your doctor may tell you to reduce the dose of sulfonylurea or insulin. To see the warning signs of low blood sugar, see section 4. If you are also taking a sulfonylurea (such as glimepiride or glibenclamide) or insulin, your doctor may ask you to have your blood sugar levels checked. This will help your doctor decide if it is necessary to change the dose of sulfonylurea or insulin.
- If you are taking insulin, your doctor will tell you how to reduce the dose of insulin and recommend that you check your blood sugar level more frequently to avoid hyperglycemia (high blood sugar) and diabetic ketoacidosis (a complication of diabetes that occurs when the body cannot break down glucose because there is not enough insulin).
- Warfarin or other anticoagulant medicines. You may need to have more frequent blood tests to check your blood clotting.
Pregnancy and breastfeeding
Tell your doctor if you are pregnant, think you may be pregnant, or are planning to have a baby. Diavic should not be used during pregnancy because it is not known if it could harm the baby.
It is not known if liraglutide passes into breast milk, so do not use this medicine during breastfeeding.
Driving and using machines
Low blood sugar (hypoglycemia) can reduce your ability to concentrate. Avoid driving or using machines if you experience symptoms of hypoglycemia. See section 4 for the warning signs of low blood sugar. Talk to your doctor for more information.
Important information about some of the ingredients of Diavic
This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
3. How to use Diavic
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are not sure, ask your doctor, pharmacist, or nurse.
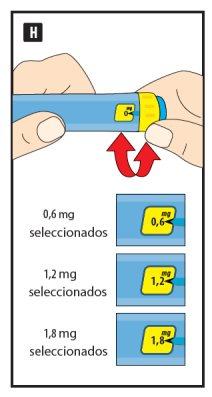
- The initial dose is 0.6 mg once a day for at least 1 week.
- Your doctor will tell you when to increase the dose to 1.2 mg once a day.
- Your doctor may tell you to further increase the dose to 1.8 mg once a day if your blood sugar is not controlled with a dose of 1.2 mg.
Do not change the dose unless your doctor tells you to.
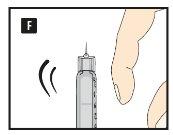
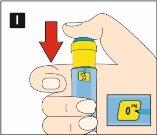
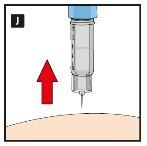
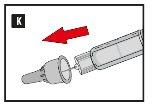
Diavic is given as an injection under the skin (subcutaneously). Do not inject into a vein or muscle. The best injection sites are the front of the thigh, the abdomen, or the upper arm. Change the injection site each day to reduce the risk of developing lumps under the skin. You can inject at any time of day, with or without food. Once you have decided on the best time of day for you, it is best to inject Diavic at the same time each day.
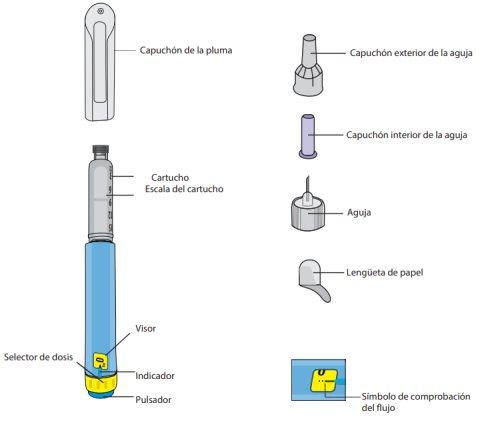
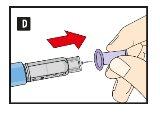
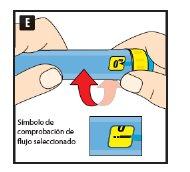
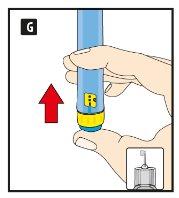
Before using the pen for the first time, your doctor or nurse will show you how to use it.
On the other side of this leaflet, you will find detailed instructions on how to use it.
If you use more Diavic than you should
If you use more Diavic than you should, talk to your doctor immediately. You can also call the Toxicology Information Service, phone 91 562 04 20, stating the medicine and the amount taken. You may need medical treatment. You may experience nausea, vomiting, diarrhea, or low blood sugar (hypoglycemia). See the warning signs of low blood sugar in section 4.
If you forget to use Diavic
If you forget a dose, use Diavic as soon as you remember.
However, if it is more than 12 hours since you should have used Diavic, skip the missed dose. Give the next dose at the usual time the next day.
Do not use a double dose or increase the dose the next day to make up for the missed dose.
If you stop using Diavic
Do not stop using Diavic without talking to your doctor. If you stop, your blood sugar levels may increase.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Common: may affect up to 1 in 10 people
- Hypoglycemia (low blood sugar). The warning signs of low blood sugar may appear suddenly and include: cold sweat, cool pale skin, headache, fast heartbeat, nausea, excessive hunger, vision problems, drowsiness, feeling weak, nervousness, anxiety, confusion, difficulty concentrating, and shakiness. Your doctor will tell you how to treat low blood sugar and what to do if you notice these warning signs. This is more likely to happen if you are also using a sulfonylurea or insulin. Your doctor may reduce your dose of these medicines before you start using Diavic.
Rare: may affect up to 1 in 1,000 people
- A severe allergic reaction (anaphylactic reaction) with additional symptoms such as breathing problems, swelling of the throat and face, fast heartbeat, etc. If you notice any of these symptoms, seek medical help immediately and talk to your doctor as soon as possible.
- Intestinal obstruction. A severe form of constipation with additional symptoms such as stomach pain, bloating, vomiting, etc.
Very rare: may affect up to 1 in 10,000 people
- Cases of pancreatitis (inflammation of the pancreas). Pancreatitis can be a serious and potentially life-threatening disease. Stop using Diavic and contact your doctor immediately if you notice any of the following serious side effects:
Severe and persistent stomach pain (in the abdomen) that may radiate to the back, as well as nausea and vomiting, as this could be a sign of pancreatitis.
Other side effects
Very common: may affect more than 1 in 10 people
- Nausea (feeling sick). This side effect usually goes away over time.
- Diarrhea. This side effect usually goes away over time.
Common: may affect up to 1 in 10 people
- Vomiting
When you start using liraglutide, you may experience loss of fluids/dehydration, for example, if you have vomiting, nausea, and diarrhea. It is important to avoid dehydration by drinking plenty of fluids.
- Headache
- Indigestion
- Stomach inflammation (gastritis). The symptoms include stomach pain, nausea, and vomiting.
- Gastroesophageal reflux disease (GERD). The symptoms include heartburn.
- Bloated stomach (abdomen) or stomach pain
- Abdominal discomfort
- Constipation
- Gas (flatulence)
- Decreased appetite
- Bronchitis
- Common cold
- Dizziness
- Fast heartbeat
- Fatigue
- Toothache
- Injection site reactions (bruising, pain, irritation, itching, and rash)
- Increased pancreatic enzymes (such as lipase and amylase).
Uncommon: may affect up to 1 in 100 people
- Allergic reactions such as itching (pruritus) and hives (urticaria)
- Dehydration, sometimes with decreased kidney function
- Feeling unwell
- Gallstones
- Inflamed gallbladder
- Change in taste
- Delayed gastric emptying.
Frequency not known (cannot be estimated from the available data):
- Lumps under the skin can occur due to the accumulation of a protein called amyloid (cutaneous amyloidosis; the frequency of this is not known).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Diavic
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and on the pen after EXP. The expiry date is the last day of the month stated.
Before use:
Store in a refrigerator (2°C–8°C). Do not freeze. Keep away from the freezer.
During use:
You can store the pen for a month if it is stored below 30 °C or in a refrigerator (between 2 °C and 8 °C), away from the freezer. Do not freeze.
When not in use, store the pen with the cap on to protect it from light.
Do not use this medicine if you notice that the solution is not clear and colorless or almost colorless.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Composition of Diavic
- The active ingredient is liraglutide. 1 ml of injectable solution contains 6 mg of liraglutide. A pre-filled pen contains 18 mg of liraglutide.
- The other components are sodium dihydrogen phosphate, propylene glycol (E1520), phenol, sodium hydroxide (for pH adjustment), concentrated hydrochloric acid (for pH adjustment), and water for injectable preparations.
Appearance and packaging of the product
Diavic is a clear and colorless or almost colorless solution, supplied with a pre-filled pen. It consists of a plunger with a bromobutyl rubber stopper and a colorless glass cartridge type I assembled in a pen-type injector, with a light blue body subset with a light blue button and a yellow dose adjustment knob with a gray cap. Each pen contains 3 ml of solution, which can deliver 30 doses of 0.6 mg, 15 doses of 1.2 mg, or 10 doses of 1.8 mg.
Diavic is available in packs of 1, 2, 3, 5, 10, or a multipack with 10 (2 packs of 5) pens.
Only some pack sizes may be marketed.
Needles are not included.
Marketing authorization holder and manufacturer
Marketing authorization holder
Sun Pharmaceutical Industries Europe B.V. Polarisavenue 87 2132JH, Hoofddorp Netherlands Manufacturer Sun Pharmaceutical Industries Europe B.V. Polarisavenue 87 2132JH, Hoofddorp Netherlands
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
|