
PHIVOR 2,500 IU Anti-Xa / 0.2 mL PRE-FILLED SYRINGES


How to use PHIVOR 2,500 IU Anti-Xa / 0.2 mL PRE-FILLED SYRINGES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Phivor and what is it used for
- What you need to know before you use Phivor
- If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
- How to use Phivor
- Possible side effects
- Storage of Phivor
- Contents of the pack and further information
Introduction
Package Leaflet: Information for the User
Phivor 2,500 IU anti-Xa/0.2 ml
Injectable solution in pre-filled syringes
Sodium bemiparin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
In this leaflet:
- What is Phivor and what is it used for
- What you need to know before you use Phivor
- How to use Phivor
- Possible side effects
- Storage of Phivor
- Contents of the pack and further information
1. What is Phivor and what is it used for
The active substance of Phivor is sodium bemiparin, which belongs to a group of medicines called anticoagulants. These medicines help prevent blood from clotting in the veins.
Phivor is used to: prevent blood clots (e.g. in the legs and/or lungs) that may appear in patients undergoing general surgery or in patients not undergoing surgical intervention but who have a moderate risk of suffering clots.
It is also used to prevent clot formation in the extracorporeal circulation circuit during haemodialysis.
2. What you need to know before you use Phivor
Do not use Phivor
- If you are allergic to sodium bemiparin, heparin or a similar product (such as enoxaparin, dalteparin, nadroparin) or to any of the other components of this medicine (listed in section 6).
- If you have had an allergic reaction after being treated with a medicine that contains heparin.
- If you are allergic to any substance derived from pigs.
- If you have Heparin-Induced Thrombocytopenia (HIT), a disease that causes a significant decrease in your platelet count (or, as a result of HIT, you suffer from another disease called Disseminated Intravascular Coagulation (DIC), in which your platelets cluster if you use Phivor).
- If you have a disease called endocarditis (inflammation of the heart walls and heart valves).
- If you have any type of disorder that causes you to bleed excessively.
- If you have a severe liver and/or pancreatic function disorder.
- If you have any type of damage or injury to your internal organs that could imply a high risk of internal bleeding (e.g. active stomach ulcers, cerebral aneurysms [inflammation of the walls of the brain arteries] or brain tumors).
- If you have had a cerebral haemorrhage.
- If you have had or have an injury or are going to be operated on in the brain, spinal cord, eyes and/or ears.
- If you are using Phivor, you will not be able to receive epidural or spinal anaesthesia (an anaesthetic injected into the spinal cord) because it could be dangerous. Therefore, make sure your doctor knows that you are using Phivor before any operation.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Phivor
- If you are ill with liver disease.
- If you are ill with kidney disease, your doctor may consider carrying out special monitoring.
- If your blood pressure is high and/or difficult to control.
- If you have ever had a stomach ulcer that is no longer active.
- If you have thrombocytopenia, a disease in which there are fewer platelets than normal in the blood, which causes bruising and easy bleeding.
- If you have kidney and/or bladder stones.
- If you have any type of disease that causes you to bleed easily.
- If you have eye problems due to problems with your blood vessels.
- If you have diabetes.
- If your test results have shown that you have high potassium levels in your blood.
- Make sure your doctor knows that you are using Phivor if you are going to have a lumbar puncture (a puncture in the lower back for analysis).
Using Phivor with other medicines
Consult your doctor if you think you may be using:
- Any medicine that is injected into the muscle, because these injections should be avoided while you are being treated with Phivor.
- Other anticoagulants such as warfarin and/or acenocoumarol (vitamin K antagonists) to treat and/or prevent blood clots.
- Non-steroidal anti-inflammatory drugs, such as ibuprofen, for example, for arthritis.
- Corticosteroids such as prednisolone, to treat inflammatory diseases, such as arthritis.
- Platelet inhibitors, such as aspirin, ticlopidine or clopidogrel, to prevent blood clots.
- Medicines that may increase potassium levels in the blood, such as some diuretics and antihypertensives (used to reduce blood pressure).
- Medicines to increase blood volume, such as dextran.
- An injectable medicine for heart problems called nitroglycerin.
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines, including those obtained without a prescription.
Special tests you may need
- Some patients may need to have their platelet levels controlled. Your doctor will decide if it is necessary and when (e.g. before starting treatment, on the first day of treatment, then every 3 or 4 days and at the end of treatment).
- If you have certain diseases (diabetes, kidney disease) or if you are taking medicines to prevent potassium loss, your doctor may control your potassium levels in your blood.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Phivor does not affect your ability to drive and use machines.
3. How to use Phivor
Follow your doctor's instructions for administering this medicine exactly. If you are in doubt, consult your doctor or pharmacist.
The recommended dose is:
Prevention of thromboembolic disease in patients undergoing general surgery:
- Phivor 2,500 IU is usually administered by a doctor or nurse via the subcutaneous route (injected under the skin, usually in a skin fold of the abdomen or in the upper part of the hip). Before or after the intervention, a dose of the product (the contents of a syringe) will be administered. On subsequent days, a dose (the contents of a syringe) will be administered every day. Your doctor will tell you how long you should be given this medicine.
Prevention of coagulation in the extracorporeal circulation circuit during haemodialysis:
- In patients undergoing haemodialysis sessions, Phivor 2,500 IU is usually administered by injecting a single dose in the form of a bolus (the contents of a syringe) into the arterial line of the dialysis circuit.
Phivor is injected under the skin, usually in a skin fold on one side of the waist (abdomen) or in the upper part of the hip. Normally, your doctor or nurse will give you the injection in the hospital. You may need to continue receiving Phivor when you go home.
- This medicine should never be injected into a muscle or mixed with any other injection.
- It is usually administered once a day.
- Your doctor will tell you how long you should be given this medicine (usually 7-10 days).
- If your doctor has told you that you can inject this medicine yourself, follow your doctor's instructions carefully (see section "How do I inject Phivor?").
Elderly(from 65 years old)
They usually receive the same dose as other adult patients. If you have liver or kidney problems, please inform your doctor, it is possible that they will decide to monitor you very closely.
Use in children(under 18 years old)
Phivor is not recommended for children.
How do I inject Phivor?
Phivor should never be injected into a muscle because it could cause bleeding inside the muscle. Before you give yourself your first injection, you should receive instructions on the correct use of this medicine and on the correct injection technique. These instructions should be given by a doctor or another qualified healthcare professional.
You should follow these steps:
- Wash your hands well and sit or lie down in a comfortable position.
- Choose a waist area that is at least 5 centimeters from the navel and from any scar or bruise, and clean the skin of that area well.
- Use different sites for injection each day, for example, first on the left side and the next time on the right.
- Remove the cap that covers the needle of the Phivor syringe.
- To keep the needle sterile, make sure it does not touch anything.
- The pre-filled syringe is ready to use.
- Before injection, do not push the plunger to eliminate air bubbles, as you may lose medicine.
- Hold the syringe with one hand and with the other, using your index and thumb fingers, hold a pinch of the cleaned skin area to form a fold.
- Insert the entire needle into the skin fold, keeping the syringe as upright as possible over the body surface, at a 90-degree angle.
- Push the plunger, making sure you keep the skin fold in the same position until the plunger is all the way down.
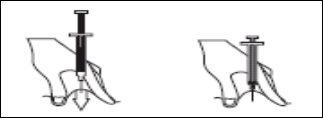
- Remove the syringe from the injection site, keeping your finger on the plunger and the syringe upright. Release the skin fold.
- Do not rub the skin where the injection was given. This will help prevent bruising.
- Do not try to put the cap back on the syringe. Immediately dispose of the syringe by throwing it into the nearest sharps container (needle first), close the container well with the lid and keep it out of the reach of children.
- If you think the effect of Phivor 2,500 IU is too strong (e.g. because you experience unexpected bleeding) or too weak (e.g. because the dose does not seem to work) tell your doctor or pharmacist.
In some pack sizes, the pre-filled syringe may be combined with a safety device that will be activated after injection to reduce the risk of needlestick injuries.
For syringes with a safety device: point the needle away from you and anyone else present, activate the safety system by firmly pressing on the plunger. The protective cover will automatically cover the needle and an audible click will confirm the activation of the device.
Immediately dispose of the syringe by throwing it into the nearest sharps container (needle first), close the container well with the lid and keep it out of the reach of children.
If you use more Phivor than you should
You may experience some type of bleeding. In this case, consult your doctor or go to the emergency department of the nearest hospital, accompanied by this leaflet.
In case of overdose or accidental administration, consult the Toxicology Information Service. Telephone 91 562 04 20.
If you forget to use Phivor
Do not take a double dose to make up for the forgotten dose. Consult your doctor as soon as possible to find out what to do.
If you stop using Phivor
Always consult your doctor before stopping this medicine.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Phivor and consult your doctor or nurse (or go immediately to the emergency department of the nearest hospital) if you experience any of the following side effects:
Common(may affect up to 1 in 10 people):
- Unusual or unexpected bleeding, for example, blood in the urine or stools, which may cause haemorrhagic anaemia.
Rare(may affect up to 1 in 1,000 people):
- Severe decrease in platelet count (type II thrombocytopenia), which can lead to bruising, bleeding from the gums, nose and mouth, and rashes.
- Skin damage (necrosis) at the injection sites.
- Intra-spinal haematomas after spinal or lumbar anaesthesia (back pain, numbness and loss of strength or sensation in the legs, incontinence in the intestine or bladder). These haematomas can cause different degrees of disability, including prolonged or permanent paralysis.
- Severe allergic reactions (fever, shivering, difficulty breathing, swelling of the vocal cords, dizziness, sweating, urticaria, rash, itching, low blood pressure, flushing, fainting, bronchial constriction, laryngeal swelling).
Other side effects:
Very common(affect more than 1 in 10 people):
- Bruises, spots on the skin, itching and some pain in the areas where you injected the medicine.
Common(may affect up to 1 in 100 people):
- A slight and transient increase in certain liver enzymes (transaminases) in the blood, which may appear in blood tests.
Uncommon(may affect up to 1 in 1,000 people):
- Mild allergic reactions on the skin (rash, skin rash, urticaria, pruritus, hives).
- Slight and transient decrease in platelet count (type I thrombocytopenia), which may appear in blood tests.
Frequency not known(cannot be estimated from the available data):
- Hyperkalaemia (increased potassium levels in the blood).
- Bone fragility (osteoporosis) associated with prolonged treatment with heparin.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Phivor
Keep this medicine out of the sight and reach of children.
Do not store above 30°C. Do not freeze.
Do not use this medicine if you notice:
- The protective packaging is open.
- The protective packaging is damaged.
- The medicine contained in the syringe is cloudy.
- Small particles in the medicine.
Once the blister pack containing the syringe is opened, the medicine should be used immediately.
Expiry date:
Do not use this medicine after the expiry date stated on the packaging.
The expiry date is the last day of the month indicated.
Disposal
This medicine is presented in single-use syringes.
Dispose of used syringes in a sharps container.
Do not store them after use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need at the pharmacy's SIGRE point. This will help protect the environment.
6. Contents of the pack and further information
Composition of Phivor
The active substance is: Sodium bemiparin 2,500 IU
The other components are: Water for injections.
Appearance of the product and pack contents
The medicine contained in the syringes is a clear, colourless or slightly yellowish solution, without visible particles.
Phivor 2,500 IU is available in packs of 2, 10, 30 and 100 pre-filled syringes. Each syringe contains 0.2 ml of solution. Each 0.2 ml syringe provides a dose of sodium bemiparin of 2,500 IU.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
GINELADIUS, S.L.
C/ Rufino González 50, 28037 Madrid - Spain
Manufacturer
ROVI Pharma Industrial Services, S.A.
C/ Julián Camarillo, 35
28037 MADRID
LABORATORIOS FARMACÉUTICOS ROVI, S.A.
C/ Julián Camarillo, 35
28037 MADRID
This medicine is authorised in the Member States of the European Economic Area under the following names:
Ivor:Austria, Greece, Italy, Portugal
Zibor:Czech Republic, Estonia, Hungary, Ireland, Latvia, Lithuania, Poland, Slovakia, Slovenia, United Kingdom
Phivor:Spain
Date of last revision of this leaflet: 05/2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PHIVOR 2,500 IU Anti-Xa / 0.2 mL PRE-FILLED SYRINGESDosage form: INJECTABLE, 10000/IUActive substance: bemiparinManufacturer: Laboratorios Farmaceuticos Rovi S.A.Prescription requiredDosage form: INJECTABLE, 12,500 IUActive substance: bemiparinManufacturer: Laboratorios Farmaceuticos Rovi S.A.Prescription requiredDosage form: INJECTABLE, 2500 IUActive substance: bemiparinManufacturer: Laboratorios Farmaceuticos Rovi S.A.Prescription required
Online doctors for PHIVOR 2,500 IU Anti-Xa / 0.2 mL PRE-FILLED SYRINGES
Discuss questions about PHIVOR 2,500 IU Anti-Xa / 0.2 mL PRE-FILLED SYRINGES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















