
PEDMARQSI 80 mg/mL SOLUTION FOR INFUSION

How to use PEDMARQSI 80 mg/mL SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Pedmarqsi 80 mg/ml Solution for Infusion
Sodium Thiosulfate
Read all of this leaflet carefully before you or your child start taking this medicine because it contains important information.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you or your child experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Pedmarqsi and what is it used for
- What you need to know before you or your child start taking Pedmarqsi
- How Pedmarqsi is administered
- Possible side effects
- Storage of Pedmarqsi
- Contents of the pack and further information
1. What is Pedmarqsi and what is it used for
Pedmarqsi contains the active substance sodium thiosulfate.
Pedmarqsi is used to reduce the risk of hearing loss caused by cisplatin, which is a cancer medicine. It is given to children and adolescents from 1 month to 18 years who are being treated with cisplatin for solid tumors that have not spread to other parts of the body.
2. What you need to know before you or your child start taking Pedmarqsi
Do not administer Pedmarqsi
if the child is:
- allergic to sodium thiosulfate or any of the other ingredients of this medicine (listed in section 6)
- a baby under 1 month of age
Warnings and precautions
Talk to your doctor or nurse before you or your child receive Pedmarqsi if the child:
- has had an allergic reaction, such as a skin rash, hives, or difficulty breathing after a previous dose of sodium thiosulfate
- has a known allergy to chemicals called sulfites, which may mean that you or the child are more likely to have an allergic reaction to this medicine
- has kidney problems or severe kidney disease
- needs a low-salt diet due to another illness
Other medicines and Pedmarqsi
Tell your doctor or nurse if you or your child are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
This medicine must not be administered if you or your daughter are pregnant (or might be pregnant) or if you are breastfeeding. This medicine is only given after chemotherapy with cisplatin, and cisplatin can harm your baby. Ask your doctor if you need to use contraceptive methods during treatment and for 6 months after treatment.
Pedmarqsi contains boric acid
This medicine contains boric acid, which may affect fertility when given for a long time.
Pedmarqsi contains sodium
This medicine contains 23 mg of sodium (main component of kitchen/table salt) per ml. This is equivalent to 1-2% of the safe dietary intake of sodium in children from 1 to 17 years and 12% in babies from 7 to 11 months.
3. How Pedmarqsi is administered
Before you or your child receive this medicine, you will be given anti-nausea medicines to help prevent vomiting.
This medicine is a solution that a doctor or nurse will give you through a vein (by infusion). This is usually done through a tube inserted into a vein in the chest, known as a central line. The infusion is given over 15 minutes. Treatment starts 6 hours after the end of the cisplatin dose.
The dose of this medicine is calculated based on your size (body surface area) in m2, which is calculated from your height and weight. The recommended dose for those who weigh 10 kg or more is 12.8 g per m2; lower doses are given to those who weigh less than 10 kg. Your doctor will determine the dose that is right for you or your child.
If you or your child receive more Pedmarqsi than you should
Because the dose is calculated and controlled by healthcare professionals, it is unlikely that you or your child will receive the wrong amount. In case of overdose, you or your child may experience nausea, vomiting, changes in sodium, phosphate, or potassium levels in the blood, changes in blood pressure, or blood acidity (metabolic acidosis) that can cause nausea, vomiting, drowsiness, and difficulty breathing. Your doctor may give you or your child symptomatic treatment for these side effects.
If you have any other questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
If you or your child experience a severe allergic reaction to this medicine with symptoms such as skin rash, chest tightness, wheezing, difficulty breathing, or feeling of cold, tell your doctor or nurse immediately.
Other side effects
Other side effects seen with this medicine are usually mild. The side effects that you or your child may experience are:
Very common(may affect more than 1 in 10 people)
- Nausea
- Vomiting
- Decrease in phosphate or potassium levels in the blood
- Increase in sodium levels in the blood
Common(may affect more than 1 in 100 people)
- Increase or decrease in blood pressure
- Decrease in calcium levels in the blood
- Blood acidity (metabolic acidosis) that can cause nausea, vomiting, drowsiness, and difficulty breathing
Reporting of side effects
If you or your child experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Pedmarqsi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial after CAD. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Pedmarqsi
- The active substance is sodium thiosulfate, in anhydrous form.
- The other ingredients are:
- boric acid (0.25 mg/ml)
- water for injections
- hydrochloric acid and sodium hydroxide for pH adjustment (see section 2; Pedmarqsi contains sodium).
Appearance and pack contents
This medicine is a solution for infusion.
This medicine is a clear and colorless sterile solution supplied in clear glass vials sealed with a rubber stopper and a flip-off cap made of aluminum. Each carton contains one vial.
Marketing authorization holder and manufacturer
Norgine B.V.
Antonio Vivaldistraat 150
1083 HP Amsterdam
Netherlands
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
----------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Dosage and administration
Timing of administration in relation to cisplatin
The timing of sodium thiosulfate administration in relation to cisplatin chemotherapy is critical.
If sodium thiosulfate is administered:
- less than 6 hours after the end of cisplatin infusion: it may reduce the effectiveness of cisplatin against the tumor
- more than 6 hours after the end of cisplatin infusion: it may not be effective in preventing ototoxicity.
Use sodium thiosulfate only after a cisplatin infusion that has lasted 6 hours or less. Do not use sodium thiosulfate if:
- the cisplatin infusion lasts more than 6 hours or
- a subsequent cisplatin infusion is planned within the next 6 hours.
When cisplatin is administered on consecutive days, ensure a minimum interval of 6 hours between the sodium thiosulfate infusion and the next cisplatin infusion.
After the end of the cisplatin infusion:
- Administer a highly effective intravenous multi-agent antiemetic 30 minutes before sodium thiosulfate administration, i.e., 5.5 hours after the end of cisplatin infusion
- This medicine is a ready-to-use solution for infusion
- Prepare the necessary ml of sodium thiosulfate, 80 mg/ml, in a syringe or add it to an empty sterile infusion bag
- Stop the cisplatin hydration fluid and flush the line with 0.9% sodium chloride
- Infuse sodium thiosulfate over 15 minutes (6 hours after the end of cisplatin infusion)
- Flush the line with 0.9% sodium chloride and resume cisplatin hydration immediately after.
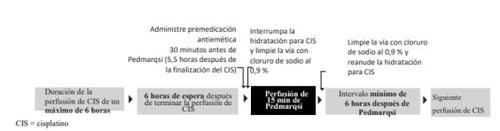
Refer to the section "Timing of administration in relation to cisplatin" for crucial information on the timing of sodium thiosulfate administration.
This medicine is presented in a single-use vial containing 8 g in a concentration of 80 mg/ml. The recommended dose of sodium thiosulfate for the prevention of cisplatin-induced ototoxicity is based on body weight and normalized according to body surface area as shown in the following table:
Body weight | Dose | Volume |
>10 kg | 12.8 g/m2 | 160 ml/m2 |
5 to 10 kg | 9.6 g/m2 | 120 ml/m2 |
<5 kg< p> | 6.4 g/m2 | 80 ml/m2 |
Instructions for use, handling, and disposal
This medicine is intended for single use. Disposal of unused parts of the solution should be done according to local regulations.
Chemical and physical stability has been demonstrated for 24 hours at controlled room temperature for the product stored in intravenous bags made of polyvinyl chloride, vinyl acetate, and polyolefin.
From a microbiological point of view, the product should be used immediately after opening. If not used immediately, the in-use storage times and conditions are the responsibility of the user and normally should not be longer than 24 hours at 2 to 8°C.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PEDMARQSI 80 mg/mL SOLUTION FOR INFUSIONDosage form: INJECTABLE, 0.5 mg/5 mlActive substance: flumazenilManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: INJECTABLE, 1 mg flumazenil/10 mlActive substance: flumazenilManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: INJECTABLE, 100 mgActive substance: sugammadexManufacturer: Merck Sharp & Dohme B.V.Prescription required
Online doctors for PEDMARQSI 80 mg/mL SOLUTION FOR INFUSION
Discuss questions about PEDMARQSI 80 mg/mL SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






