

Individualni Zestav Autostzhikavek pzhecivko Boiovim Shrodkom Truiomcim Izas-05

Ask a doctor about a prescription for Individualni Zestav Autostzhikavek pzhecivko Boiovim Shrodkom Truiomcim Izas-05

How to use Individualni Zestav Autostzhikavek pzhecivko Boiovim Shrodkom Truiomcim Izas-05
Leaflet attached to the packaging: patient information
Individual Auto-Injector Kit against Combat Toxic Agents
IZAS-05
ATROPINE Auto-Injector,
2 mg/2 mL, solution for injection (Atropine sulfate) ATROPINE + PRALIDOXIME Auto-Injector,
600 mg/2 mL + 2 mg/2 mL, solution for injection (Pralidoxime chloride + Atropine sulfate) DIAZEPAM Auto-Injector,
10 mg/2 mL, solution for injection (Diazepam)
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor, pharmacist, or nurse.
- If the patient experiences any adverse reactions, including any adverse reactions not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is the IZAS-05 kit and what is it used for
- 2. Important information before using the IZAS-05 kit
- 3. How to use the IZAS-05 kit
- 4. Possible adverse reactions
- 5. How to store the IZAS-05 kit
- 6. Package contents and other information
1. What is the IZAS-05 kit and what is it used for
IZAS-05 is a kit of three auto-injectors (ATROPINE auto-injector, PRALIDOXIME + ATROPINE auto-injector, DIAZEPAM auto-injector), intended for use in the event of poisoning with combat toxic agents from the group of paralytic-convulsive agents. The IZAS-05 kit is used to alleviate symptoms and counteract the effects of poisoning.
2. Important information before using the IZAS-05 kit
When not to use the components of the IZAS-05 kit
ATROPINE Auto-Injector
- if the patient is allergic to the active substance or any of the other ingredients of this medicine (listed in section 6),
- if the patient has narrow-angle glaucoma,
- if the patient has diseases that cause disorders of urinary tract patency (e.g., prostate hypertrophy) or gastrointestinal tract.
PRALIDOXIME + ATROPINE Auto-Injector
- there are no known absolute contraindications to the use of pralidoxime chloride,
- do not use if the patient is allergic to pralidoxime chloride, or any of the other ingredients of this medicine (listed in section 6), and in other situations where the risk clearly outweighs the potential benefits of using the medicine.
When poisoning with combat toxic agents with paralytic-convulsive action, the relevance of the listed contraindications should be assessed, considering both the possible risk and the benefits for the patient resulting from the use of the auto-injectors included in the IZAS-05 kit.
Warnings and precautions
Before starting to use the IZAS-05 kit, you should discuss it with your doctor or nurse, if possible.
ATROPINE Auto-Injector
Atropine should be administered with caution to patients:
- over 40 years old, due to the possibility of prostate hypertrophy and the occurrence of urinary tract patency disorders;
- with respiratory diseases, as atropine may exacerbate shortness of breath;
- with hyperthyroidism, hypertension, tachyarrhythmia, congestive heart failure, angina pectoris, autonomic nervous system neuropathy, gastrointestinal diseases (e.g., gastric ulcer, esophageal reflux);
- with renal and/or hepatic function disorders.
PRALIDOXIME + ATROPINE Auto-Injector
- Pralidoxime chloride is not effective in the treatment of poisoning with phosphorus, inorganic phosphates, or organophosphorus compounds without anticholinesterase action.
- Pralidoxime chloride is not indicated as an antidote in the case of poisoning with pesticides belonging to the carbamate class, as it may enhance their toxic action.
DIAZEPAM Auto-Injector
Particular caution should be exercised when using diazepam in patients:
- of advanced age,
- with liver failure,
- with chronic pulmonary insufficiency,
- with brain damage resulting from, for example, injury, atherosclerosis of blood vessels, stroke,
- in a severe condition, especially with cardiac and respiratory disorders,
- with chronic respiratory insufficiency,
- addicted to drugs or alcohol,
- who have lost loved ones and are in a period of mourning,
- suffering from phobias and obsessions,
- suffering from chronic psychoses.
Tolerance After using diazepam for several weeks, its effectiveness may decrease. Dependence Long-term use of diazepam can lead to psychological and physical dependence. The risk of developing dependence increases with increasing dose and treatment time and is higher in patients addicted to alcohol or drugs, as well as in patients with personality disorders. Withdrawal symptoms In the event of sudden withdrawal of the drug, the patient may experience withdrawal symptoms, such as: headaches, muscle pain, increased anxiety, tension, excitement, restlessness, disorientation, sleep disturbances, irritability. In more severe cases, the following may occur: loss of sense of reality, personality disorders, hypersensitivity to sound, touch, light, noise, feeling of tingling and numbness of limbs, hallucinations and delusions, epileptic seizures. Rebound phenomenon and anxiety During diazepam withdrawal, a transient recurrence of intensified symptoms that were the reason for using the drug (so-called rebound phenomenon) may occur. These symptoms are often accompanied by mood changes, anxiety, sleep disturbances, and insomnia. To minimize the risk of these symptoms, it is recommended to gradually reduce the dose of the drug. Anterograde amnesia (inability to remember events after taking the drug) Diazepam may cause anterograde amnesia (difficulty learning and remembering new information – new data is not permanently stored). This condition usually occurs within a few hours of taking the drug, especially in high doses. Psychotic and paradoxical reactions When using benzodiazepines, reactions such as anxiety, agitation, irritability, aggression, delusions, fury attacks, nightmares, hallucinations, psychoses, inappropriate behavior, and other behavioral disorders have been reported. If such symptoms occur, the use of the drug should be discontinued. The risk of these adverse reactions is higher in children and the elderly. Use in depression Before using diazepam, you should inform your doctor about any mental illnesses. Patients with symptoms of depression or anxiety associated with depression should use several drugs simultaneously. Administering diazepam to patients with depression alone may exacerbate symptoms of depression, including suicidal thoughts.
Children and adolescents
Individual Auto-Injector Kit against Combat Toxic Agents IZAS-05 is not intended for use in children.
IZAS-05 and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
ATROPINE Auto-Injector
Medicines that enhance the effects of atropine:
- tricyclic antidepressants,
- antispasmodic drugs (used, for example, in irritable bowel syndrome),
- drugs used in Parkinson's disease,
- certain antihistamines (classified as first-generation),
- phenothiazines (drugs used in mental illnesses),
- disopyramide, quinidine (drugs used in heart diseases).
Atropine, by delaying gastric emptying, may cause slowed or accelerated absorption of some orally administered drugs.
PRALIDOXIME + ATROPINE Auto-Injector
Using pralidoxime in combination with atropine may cause earlier signs of atropine use than when atropine is used as a single drug. There have been reports of excitement and manic behavior after regaining consciousness. After intramuscular injection of the drug, the following may occur:
- increased heart rate,
- dry mouth
- blurred vision
- floaters in the visual field
- anxiety
- headache
- difficulty urinating
DIAZEPAM Auto-Injector
Diazepam and other concomitantly used drugs may mutually affect their action. In particular, this applies to the following drugs:
- fluvoxamine, fluoxetine, and other drugs used in the treatment of mental illnesses;
- drugs used in insomnia;
- drugs used in allergic diseases that may cause drowsiness;
- drugs used in epilepsy (e.g., carbamazepine, phenytoin, hydantoin);
- muscle relaxants;
- levodopa (a drug used in Parkinson's disease);
- drugs used in gastric and duodenal ulcer disease (e.g., cimetidine, omeprazole, cisapride);
- ketokonazole (an antifungal drug);
- strong painkillers (called opioid painkillers, e.g., morphine, buprenorphine);
- rifampicin (an antibiotic).
Theophylline and smoking accelerate the metabolism of diazepam.
DIAZEPAM Auto-Injector with alcohol
Using the drug in combination with alcohol may enhance the sedative effect. It is not recommended to take diazepam and alcohol simultaneously.
Pregnancy, breastfeeding, and fertility
ATROPINE Auto-Injector
The drug may be used during pregnancy only in cases where the benefit to the mother outweighs the potential risk to the fetus.
PRALIDOXIME + ATROPINE Auto-Injector
Pralidoxime chloride can be administered to pregnant women only in cases of absolute necessity, when the potential risk is acceptable due to the expected clinical benefits.
DIAZEPAM Auto-Injector
Diazepam should not be used in pregnant women, especially during the first and third trimesters, and during breastfeeding, unless the doctor decides that the use of the drug is necessary.
Driving and operating machinery
Atropine sulfate has a significant impact on the ability to drive vehicles and operate machinery. During the use of the drug and 24 hours after its administration, you should not drive vehicles or perform tasks that require good psychophysical fitness. Diazepam has a significant impact on the ability to drive vehicles and operate machinery. During the use of the drug and 24 hours after its administration, you should not drive vehicles or perform tasks that require good psychophysical fitness. The IZAS-05 kit contains benzyl alcohol, so it should not be administered to premature infants or newborns. Benzyl alcohol present in the drug may cause poisoning and allergic reactions in infants and children up to 3 years old. The IZAS-05 kit contains ethanol.This should be taken into account when using it in pregnant or breastfeeding women, children, and individuals at high risk, such as patients with liver disease, epilepsy, or alcoholism. The IZAS-05 kit contains sodium benzoate, which may increase the risk of jaundice in newborns.
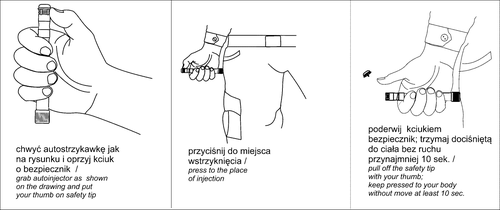
3. How to use the IZAS-05 kit
The drugs should be used in the event of exposure to combat toxic agents derived from organophosphorus compounds from the group of paralytic-convulsive agents. ATROPINE Auto-Injector: remove from the outer packaging, press the yellow end against the injection site, lift the red safety shield, and hold still for 10 seconds (self-administration). PRALIDOXIME + ATROPINE Auto-Injector: remove from the outer packaging, press the yellow end against the injection site, lift the red safety shield, and hold still for 10 seconds (self-administration). DIAZEPAM Auto-Injector: remove from the outer packaging, press the gray end against the injection site, lift the red safety shield, and hold still for 10 seconds (mutual assistance).
4. Possible adverse reactions
Like any medicine, the drugs in the IZAS-05 kit may cause adverse reactions, although they do not occur in everyone.
ATROPINE Auto-Injector
Adverse reactions depend on the size of the administered dose and usually disappear after discontinuation of the drug. After administration of relatively small doses: Very common adverse reactions (may occur in more than 1 in 10 people):
- decreased saliva secretion and dry mouth
- constipation
- reflux
- loss of taste
- nausea
- vomiting
- bloating
- decreased secretion of bronchial mucus, which may cause thickening of the mucus and formation of a bronchial plug that is difficult to remove from the airways
- decreased sweating (anhidrosis)
- hives and rash, sometimes with peeling of the skin
- visual disturbances (pupil dilation, accommodation disorders, photophobia, blurred vision)
- these reactions are exacerbated by increasing the dose of atropine.
After administration of large doses of atropine, the following have been reported: Common adverse reactions (may occur in less than 1 in 10 people):
- inhibition of excessive stimulation of the vagus nerve, and consequently, acceleration of heart rate with possible occurrence of atrial flutter or fibrillation, atrioventricular dissociation, and ventricular extrasystoles
- urinary retention and constipation
- inhibition of gastric secretion
- hallucinations or excitement, confusion and lack of coordination, disorientation – especially in elderly patients
- hyperthermia
- flushing of the face
Uncommon adverse reactions (may occur in less than 1 in 100 people):
- psychotic reactions
Rare adverse reactions (may occur in less than 1 in 1,000 people):
- allergic reactions
- drowsiness, fatigue
Very rare adverse reactions (may occur in less than 1 in 10,000 people):
- anaphylaxis
- atrial arrhythmia with ventricular fibrillation
- angina pectoris
- hypertensive crisis
- increased intraocular pressure
Frequency not known (frequency cannot be estimated from available data):
- headaches
- nervousness
- dizziness
- insomnia
- ataxia
PRALIDOXIME + ATROPINE Auto-Injector
When pralidoxime and atropine are administered together, signs of atropine use may occur earlier than when atropine is used as a single drug. There have been reports of excitement and manic behavior after regaining consciousness. After intramuscular injection of the drug, the following may occur:
- increased heart rate,
- dry mouth
- blurred vision
- floaters in the visual field
- anxiety
- headache
- difficulty urinating
DIAZEPAM Auto-Injector
After intramuscular injection of the drug, the following may occur:
- pain at the injection site,
- redness at the injection site.
The following may also occur: feeling of fatigue, drowsiness, and muscle weakness. Using the drug (even in therapeutic doses) may lead to dependence. Abuse of benzodiazepine drugs has been observed. Rarely (in 1 to 10 people out of 10,000), other adverse reactions have been observed, such as:
- confusion, decreased emotional reactivity, decreased level of consciousness, impaired coordination, tremors,
- anterograde amnesia (the patient does not remember events that occurred after taking diazepam)
- depression,
- double or blurred vision,
- speech or unclear speech disorders,
- gastrointestinal disorders, nausea, dry mouth or excessive salivation, constipation, increased appetite,
- headache, dizziness,
- decreased blood pressure, changes in heart rate, depressive circulation (significant slowing of heart action), changes in blood count (visible in the so-called morphology test),
- urinary incontinence or retention,
- increased or decreased libido,
- skin allergic reactions.
Very rarely (in less than 1 person out of 10,000), the following have been observed:
- increased activity of certain enzymes (transaminases and alkaline phosphatase)
- jaundice
- cases of cardiac arrest.
After using diazepam (especially in children and elderly patients), the following may occur: anxiety, agitation, hallucinations, changes in behavior, aggression, nightmares, psychoses (so-called paradoxical reactions). In elderly patients and weakened individuals, exacerbated adverse reactions may occur. After using diazepam, latent depression may be revealed. If any adverse reactions occur, including any adverse reactions not listed in the leaflet, you should consult your doctor, pharmacist, or nurse.
Reporting adverse reactions
If any adverse reactions occur, including any adverse reactions not listed in the leaflet, you should inform your doctor, pharmacist, or nurse. Adverse reactions can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49-21-301, Fax: +48 22 49-21-309, Website: https://smz.ezdrowie.gov.pl. Adverse reactions can also be reported to the marketing authorization holder. By reporting adverse reactions, more information can be collected on the safety of the use of the drug.
5. How to store the IZAS-05 kit
The drug should be stored in a place invisible and inaccessible to children. Do not use this drug after the expiration date stated on the box and label after: EXP. The expiration date means the last day of the given month. Store at a temperature below 25°C. Do not freeze.
6. Package contents and other information
What does the IZAS-05 kit contain
ATROPINE Auto-Injector
The active substance is atropine sulfate,2 mL solution for injection contains 2 mg of atropine sulfate. The other ingredients are: diluted hydrochloric acid (to adjust pH), water for injections.
PRALIDOXIME + ATROPINE Auto-Injector
First chamber:
The active substance is atropine sulfate,2 mL solution for injection contains 2 mg of atropine sulfate. The other ingredients are: diluted hydrochloric acid (to adjust pH), water for injections.
Second chamber:
The active substance is pralidoxime chloride, 2 mL solution for injection contains 600 mg of pralidoxime chloride. The other ingredients are: benzyl alcohol, glycine, water for injections.
DIAZEPAM Auto-Injector
The active substance of the drug is diazepam,2 mL solution for injection contains 10 mg of diazepam. The other ingredients are: benzyl alcohol, ethanol, propylene glycol, sodium benzoate (E 211), glacial acetic acid, 10% acetic acid, water for injections.
What the IZAS-05 kit looks like and what the packaging contains
Individual Auto-Injector Kit against Combat Toxic Agents IZAS-05 contains three auto-injectors:
ATROPINE Auto-Injector(small) –length 115 mm, consists of a yellow body and yellow cap, and a red safety shield.
DIAZEPAM Auto-Injector(small) –length 115 mm, consists of a gray body and gray cap, and a red safety shield.
PRALIDOXIME + ATROPINE Auto-Injector(large) –length 142 mm, consists of a brown body and yellow cap, and a red safety shield.
Follow the instructions below:
The auto-injectors that make up the IZAS-05 kit are placed in a green plastic case.
Marketing authorization holder and manufacturer
Zakład Produkcji Sprzętu Medycznego Ravimed Sp. z o.o. ul. Polna 54 05-119 Łajski
Date of the last update of the leaflet:
- Country of registration
- Prescription requiredYes
- ImporterZakład Produkcji Sprzętu Medycznego RAVIMED Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Individualni Zestav Autostzhikavek pzhecivko Boiovim Shrodkom Truiomcim Izas-05Dosage form: Solution, 100 mg/mlActive substance: acetylcysteineManufacturer: Lek Pharmaceuticals d.d.Prescription requiredDosage form: Solution, 0.1 mg/mlActive substance: flumazenilManufacturer: B. Braun Melsungen AGPrescription requiredDosage form: Solution, 0.1 mg/mlActive substance: flumazenilManufacturer: Fresenius Kabi Austria GmbHPrescription required
Alternatives to Individualni Zestav Autostzhikavek pzhecivko Boiovim Shrodkom Truiomcim Izas-05 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Individualni Zestav Autostzhikavek pzhecivko Boiovim Shrodkom Truiomcim Izas-05 in España
Online doctors for Individualni Zestav Autostzhikavek pzhecivko Boiovim Shrodkom Truiomcim Izas-05
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Individualni Zestav Autostzhikavek pzhecivko Boiovim Shrodkom Truiomcim Izas-05 – subject to medical assessment and local rules.














