OXERVATE 20 micrograms/ml eye drops solution

How to use OXERVATE 20 micrograms/ml eye drops solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
OXERVATE 20 micrograms/ml eye drops, solution
Cenegermin
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What OXERVATE is and what it is used for
- What you need to know before you use OXERVATE
- How to use OXERVATE
- Possible side effects
- Storing OXERVATE
- Contents of the pack and other information
1. What OXERVATE is and what it is used for
OXERVATE contains the active substance cenegermin, which is a type of nerve growth factor (a human protein) that is normally present on the surface of the eye.
OXERVATE is used to treat adults with moderate or severe neurotrophic keratitis, which is a disease affecting the cornea (the transparent layer on the front of the eye) and causing defects on the outer surface of the cornea that do not heal naturally or corneal ulcers.
OXERVATE is indicated to allow the cornea to heal.
2. What you need to know before you use OXERVATE
Do not use OXERVATE:
- if you are allergic to cenegermin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Use this medicine only in the affected eye.
Consult your doctor beforestarting to use this medicine:
- If you have an eye infection, as the infection needs to be treated first. If you have an eye infection whileusing OXERVATE, stop the treatment immediately and consult your doctor as soon as possible.
- If you have eye cancer, as this medicine could make it worse.
- If you are using any eye drops that contain corticosteroids (e.g. to treat eye inflammation) or preservatives (e.g. benzalkonium chloride, polyquaternium-1, benzododecinium bromide or cetrimide). Eye drops containing these substances may slow down or hinder the healing of the eye and their use should be avoided during treatment with this medicine.
Treatment with OXERVATE may cause mild to moderate eye discomfort, such as eye pain. If you experience a severe eye reaction, ask your doctor for advice.
Contact lenses may hinder the correct use of this medicine. If you wear contact lenses, remove them before using this medicine and wait 15 minutes afterusing this medicine before putting them back on.
Children and adolescents
This medicine OXERVATE should not be used in children and adolescents under 18 years of age, as there is not enough information on its use in this age group.
Other medicines and OXERVATE
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
You should wait at least 15 minutes before or afterusing OXERVATE if you use another eye drop. This will prevent one eye drop from being diluted by the other. If you are also using an eye ointment or a viscous eye drop, you should use OXERVATE firstand then wait at least 15 minutes beforeusing the other medicine.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
This medicine should not be used during pregnancy. If you are pregnant or think you may be pregnant, consult your doctor.
It is not known whether cenegermin passes into breast milk. Talk to your doctor before breast-feeding your baby, as it will be necessary to decide whether to stop breast-feeding or stop the treatment, taking into account the benefit of breast-feeding for the child and the benefit of the treatment for the mother.
Driving and using machines
Immediately after using this medicine, you may experience temporary blurred vision. If this happens, wait until your vision clears before driving or using machines.
3. How to use OXERVATE
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again.
The recommended doseis 1 drop in the affected eye, 6 times a day, with a 2-hour interval, starting in the morning (i.e. 6 drops per day over 12 hours). You should continue your treatment for 8 weeks.
Instructions for use
Follow the instructions below carefully and if there is something you do not understand, ask your doctor or pharmacist.
Ophthalmic use
You will receive a protective package containing a weekly box of OXERVATE and a separate administration device (containing the necessary medical devices for extraction and administration of the medicine).
The weekly box contains 7 vials of OXERVATE (1 vial per day of the week). Remove the weekly box of OXERVATE from the protective package and store it in the refrigerator as soon as possible (and no later than 5 hours after the pharmacist has dispensed the medicine). Since this medicine is stored in the pharmacy in a freezer, if you start treatment immediately after receiving the weekly box, you will have to wait until the first vial has thawed (which may take 30 minutes).
In the morning, take a single vial of this medicine from the refrigerator (always at the same time every morning) and prepare it as follows:
- Wash your hands.
- If you wear contact lenses, remove them before using the eye drops.
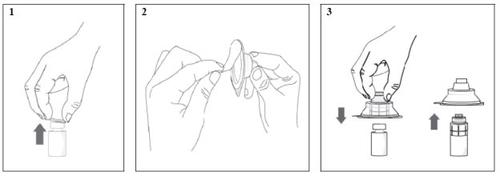
- Remove the plastic cap from the vial (image 1).
- Remove the rear part of the blister from the vial adapter (image 2).
- Without removing the vial adapter from its blister, connect it to the vial by pushing it down until it clicks into place on the neck of the vial. The tip of the vial adapter should pierce the rubber stopper of the vial. Once the adapter is correctly connected, do not remove it from the vial (image 3).
- Remove and discard the vial adapter packaging.
|
Now the OXERVATE multidose vial is ready for use (1 drop in the affected eye, every 2 hours, 6 times a day). The vial can be stored in the refrigerator or below 25 °C during the day, but do not freeze it.
To extract and administer each dose of this medicine, follow the steps below:
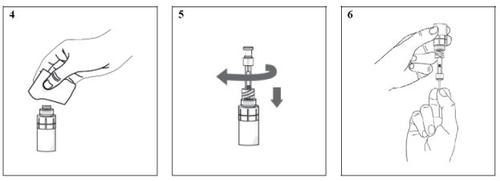
- Take a disinfectant wipe and gently clean the surface of the connector of the vial adapter (image 4). After cleaning, wait approximately 1 minute for the valve to dry.
- Take a pipette (dropper) and remove it from its protective packaging.
- Screw the pipette (clockwise) into the connector of the vial adapter (image 5).
- Check that the plunger of the pipette is fully down.
- Hold the vial upside down (with the pipette still connected) and gently pull the plunger until it stops to extract the solution into the pipette. Make sure the plunger reaches the stop (image 6).
| ||
- Check the pipette to make sure it contains the eye drop solution. Air bubbles could block the pipette and prevent it from filling properly (especially during the first extraction). If the pipette is empty, hold the vial with the pipette connected upside down, push the plunger as far as it will go and then pull it again.
- Once the pipette is filled correctly, unscrew it from the connector of the vial adapter.
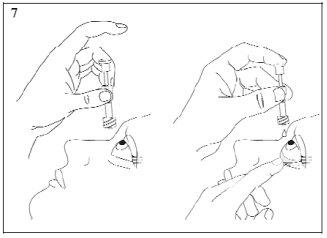
- Hold the pipette with the tip down between your middle finger and thumb, tilt your head back and place the pipette over the affected eye. Pull down the lower eyelid, exposing the pouch between the inner surface of the eyelid and the eyeball. Gently press the plunger until a single drop falls into the conjunctival sac (image 7). Make sure not to touch the eye with the tip of the pipette.
- With your head still tilted back, blink a few times to allow the medicine to cover the surface of the eye.
- Discard the used pipette immediately after use, even if there is still some liquid left in it.
- If the drop does not get into your eye, try again with a new pipette and a new disinfectant wipe.
- After each use throughout the day, put the vial back in the refrigerator (or store it below 25 °C) for the rest of the day, with the adapter still connected.
|
Repeat the procedure above (from image 4 to the end) every 2 hours, 6 times a day. Each time, use a new disinfectant wipe and a new pipette.
If you use the eye drops in both eyes, repeat the instructions above in the other eye with a new pipette (in this case, you will need to use two vials per day).
Discard the used vial at the end of each day (even if there is still some liquid left in it) and no later than 12 hours after connecting the adapter.
Each week you will receive a new supply of OXERVATE while the treatment period lasts.
To ensure the exact administration of the dose every 2 hours, you can set an alarm to remind you to take the dose.
To check that you have taken six doses at the end of each day of treatment, you should use the weekly dose administration card provided with the administration device. On this card, you should write the date of the first use of the weekly supply, the time of opening the vial (i.e. when you connect the adapter to the vial) and the time of each administration of the medicine during the week.
If you use more OXERVATE than you should
If you use more medicine than you should, rinse the affected eye with lukewarm water. Do not put in more drops until it is time for your next scheduled dose. Using more OXERVATE than recommended is unlikely to be harmful. Continue with the next scheduled dose as planned.
If you forget to use OXERVATE
Continue with the next scheduled dose as planned. Do not take a double dose to make up for forgotten doses. You can take the missed dose 2 hours after the last scheduled dose of the day, provided it is within 12 hours of opening the daily vial. Do not use more than 6 drops per day in the affected eye.
If you stop treatment with OXERVATE
The eye injury or ulcer will worsen and may cause infections or vision problems. If you intend to stop using OXERVATE, talk to your doctor first.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects occur in and around the eyes.
The following side effects have been reported:
Very common (may affect more than 1 in 10 people)
- eye pain.
Common (may affect up to 1 in 10 people)
- eye inflammation,
- eyelid pain,
- abnormal sensation and eye discomfort, including the feeling of something in the eye.
- increased tearing (this side effect may include symptoms such as eye discharge),
- eyelid inflammation, accompanied by itching and redness,
- conjunctival redness (mucous membrane covering the front of the eye and lining the inside of the eyelids),
- photosensitivity,
- eye irritation or around the eyes,
- headache.
Uncommon (may affect up to 1 in 100 people)
- excessive growth of blood vessels inside the cornea,
- corneal infection, accompanied by pus and swelling.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing OXERVATE
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and vial label after ‘EXP’. The expiry date is the last day of the month shown.
Store the weekly box containing 7 vials of OXERVATE in the refrigerator (2-8 °C).
After the adapter is connected to the vial, it can be stored in the refrigerator or below 25 °C. Discard the used vial at the end of the day (even if there is still some liquid left in it) and no later than 12 hours after connecting the vial adapter.
The pipettes included in the administration device are for single use only. Each pipette should be discarded immediately after use, even if there is still some liquid left in it.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of OXERVATE
- The active substance is cenegermin. One millilitre of OXERVATE contains 20 micrograms of cenegermin.
- The other ingredients are trehalose dihydrate, mannitol, disodium phosphate anhydrous, sodium dihydrogen phosphate dihydrate, hypromellose, macrogol 6000, L-methionine and water for injections, hydrochloric acid, sodium hydroxide and nitrogen.
Appearance and packagingOXERVATE is a clear and colourless eye drop solution.
It is supplied in multidose glass vials.
Each vial contains 1 ml of eye drop solution.
The vials are packaged in a weekly carton box, containing 7 vials.
In addition to the vial, 7 vial adapters, 42 pipettes, 42 disinfectant wipes and a dose recording card are provided. The following will be included as spare: 1 adapter, 3 pipettes and 3 disinfectant wipes.
Presentation: 7 multidose vials.
Marketing authorisation holder and manufacturerMarketing authorisation holder Dompé farmaceutici S.p.A.
Via Santa Lucia, 6
20122 Milan
Italy
Manufacturer
Dompé farmaceutici S.p.A.
Via Campo di Pile
67100 L’Aquila
Italy
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OXERVATE 20 micrograms/ml eye drops solutionDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not requiredDosage form: EYEDROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not requiredDosage form: EYE DROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not required
Online doctors for OXERVATE 20 micrograms/ml eye drops solution
Discuss questions about OXERVATE 20 micrograms/ml eye drops solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions