OSPOLOT 20 mg/ml ORAL SUSPENSION

How to use OSPOLOT 20 mg/ml ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ospolot 20 mg/ml Oral Suspension
sultiamo
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ospolot and what is it used for
- What you need to know before you take Ospolot
- How to take Ospolot
- Possible side effects
- Storing Ospolot
- Contents of the pack and other information
1. What is Ospolot and what is it used for
This medicinal product contains the active substance sultiamo, an antiepileptic used for the treatment of a certain form of epilepsy.
Ospolot is used for the treatment of CAE-RCT (childhood epilepsy with centrotemporal spikes) (previously called Rolandic epilepsy) in children and adolescents over 3 years who are non-responders/intolerant to other treatments or have no other therapeutic alternatives.
2. What you need to know before you take Ospolot
Do not take Ospolot
- if you are allergic to sultiamo, other sulfamides, or any of the other ingredients of this medicinal product (listed in section 6),
- if you have hyperthyroidism,
- if you have arterial hypertension,
- if you have acute porphyria (a congenital or acquired disorder in which the body is unable to produce sufficient red blood pigment).
Warnings and precautions
Consult your doctor before starting to take Ospolot
- if you have impaired renal function,
- if you suffer from any psychiatric disorder.
Consult your doctor immediately and request a blood test if you observe allergic reactions with fever, sore throat, skin rash with lymph node inflammation or flu-like symptoms during treatment with Ospolot. Your doctor may consider it necessary to interrupt treatment with Ospolot if you experience severe allergic reactions.
It is recommended to perform an initial check of the blood count, liver enzymes, and renal function before starting treatment with Ospolot, once a week during the first month of treatment, and subsequently once a month. After six months of treatment, it will be sufficient to perform two to four checks per year.
A small number of patients treated with antiepileptics such as sultiamo have had suicidal thoughts or thoughts of self-harm. If you ever have these thoughts, contact your doctor immediately.
Other medicines and Ospolot
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Ospolot and the following medicines or groups of medicines may interact with each other in case of combined treatment.
Combination of Ospolot and other medicines for treating epilepsy:
- Phenytoin: phenytoin blood levels may increase considerably. This combination requires close monitoring. Therefore, your doctor will perform frequent checks of your phenytoin blood levels, especially if you have impaired renal function.
- Lamotrigine: in isolated cases, an increase in lamotrigine blood levels may be observed. Therefore, lamotrigine levels should be monitored more frequently when starting this combined treatment.
- Primidone: the adverse effects of Ospolot may be potentiated. In particular, it may cause instability when walking, dizziness, and drowsiness.
- Carbamazepine: a reduction in sultiamo blood levels has been observed when taken simultaneously with carbamazepine.
When sultiamo is taken simultaneously with other carbonic anhydrase inhibitors (e.g., topiramate, used to treat epilepsy and migraine, or acetazolamide, used to treat increased intraocular pressure), the risk of adverse effects may increase due to carbonic anhydrase inhibition.
Taking Ospolot with alcohol
Do not drink alcohol during treatment with Ospolot, as alcohol may alter and potentiate the effect of Ospolot in an unpredictable manner.
When taken with alcohol, Ospolot may also cause a very unpleasant reaction, with vasodilation, pulsating headache, difficulty breathing, nausea, vomiting, palpitations, decreased blood pressure, blurred vision, confusion, shock reactions, cardiac rhythm disturbances, loss of consciousness, and convulsions. The intensity and duration of these symptoms may vary widely.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Pregnancy
There is a high risk that this medicine may harm the fetus. Therefore, do not use this medicine during pregnancy unless your doctor has specifically prescribed it for you. If you are of childbearing age and are taking Ospolot, you must use an effective contraceptive method.
Consult your doctor before stopping treatment with Ospolot. Sudden interruption of treatment or unsupervised reduction of the dose may lead to a recurrence of epileptic seizures that may harm you or the fetus.
Breast-feeding
It is unknown whether the active substance contained in Ospolot passes into breast milk. For this reason, you should nottake Ospolot during breast-feeding.
Driving and using machines
Although used as indicated, this medicine may alter your reaction capacity to such an extent that it reduces, for example, your ability to drive or use machines. This is especially applicable when combined with alcohol.
Ospolot contains methylparahydroxybenzoate sodium (E219), propylparahydroxybenzoate sodium (E217), sulfur dioxide (E220), sodium, fructose, glucose, and sucrose.
Methylparahydroxybenzoate sodium (E219) and propylparahydroxybenzoate sodium (E217) may cause allergic reactions (possibly delayed).
Sulfur dioxide (E220) may rarely cause severe hypersensitivity reactions and bronchospasm.
This medicine contains 0.0026 mg of fructose per milliliter.
Glucose and sucrose: if your doctor has told you that you have an intolerance to some sugars, consult with them before taking this medicine.
Glucose, fructose, and sucrose may damage teeth
This medicine contains less than 1 mmol of sodium (23 mg) per ml, i.e., it is essentially “sodium-free”.
3. How to take Ospolot
Treatment with Ospolot should be initiated and supervised by doctors with experience in the treatment of epilepsy.
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Dose
Usually, your doctor will indicate that you start with a low dose and gradually increase it over a week, until you reach a suitable dose for you (called the maintenance dose). The usual maintenance dose is 5 to 10 mg (0.25-0.5 ml) per kilogram of body weight and day.
It is recommended to distribute the daily dose into three single doses.
Table 1: Examples of doses for a starting dose of 2.5 mg of sultiamo per kg per day
Patient weight | Initial dose: 2.5 mg of sultiamo per kg per day | |
Dose per intake (administered 3 times a day) | Total daily dose | |
12-18 kg | 0.5-0.75 ml (equivalent to 10-15 mg of sultiamo) | 1.5-2.25 ml (equivalent to 30-45 mg of sultiamo) |
18-24 kg | 0.75-1.0 ml (equivalent to 15-20 mg of sultiamo) | 2.25-3.0 ml (equivalent to 45-60 mg of sultiamo) |
24-30 kg | 1.0-1.25 ml (equivalent to 20-25 mg of sultiamo) | 3.0-3.75 ml (equivalent to 60-75 mg of sultiamo) |
30-36 kg | 1.25-1.5 ml (equivalent to 25-30 mg of sultiamo) | 3.75-4.5 ml (equivalent to 75-90 mg of sultiamo) |
From 36 kg | 1.5 ml and above (equivalent to 30 mg of sultiamo and above) | 4.5 ml and above (equivalent to 90 mg of sultiamo and above) |
*1 ml of Ospolot oral suspension contains 20 mg of sultiamo => 0.25 ml = 5 mg of sultiamo
Table 2: Examples of doses for a maintenance dose of 5 mg of sultiamo per kg per day:
Patient weight | Maintenance dose: 5 mg of sultiamo per kg per day | |
Dose per intake (administered 3 times a day) | Total daily dose | |
12-18 kg | 1.0-1.5 ml (equivalent to 20-30 mg of sultiamo) | 3.0-4.5 ml (equivalent to 60-90 mg of sultiamo) |
18-24 kg | 1.5-2.0 ml (equivalent to 30-40 mg of sultiamo) | 4.5-6.0 ml (equivalent to 90-120 mg of sultiamo) |
24-30 kg | 2.0-2.5 ml (equivalent to 40-50 mg of sultiamo) | 6.0-7.5 ml (equivalent to 120-150 mg of sultiamo) |
30-36 kg | 2.5-3.0 ml (equivalent to 50-60 mg of sultiamo) | 7.5-9.0 ml (equivalent to 150-180 mg of sultiamo) |
From 36 kg | 3.0 ml and above (equivalent to 60 mg of sultiamo and above) | 9.0 ml and above (equivalent to 180 mg of sultiamo and above) |
*1 ml of Ospolot oral suspension contains 20 mg of sultiamo => 0.25 ml = 5 mg of sultiamo
Note:Tablets can be used for single doses of 10 ml or more.
Form and route of administration
Ospolot is indicated for oral use.
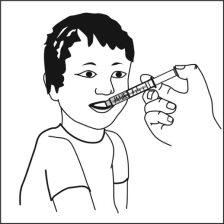
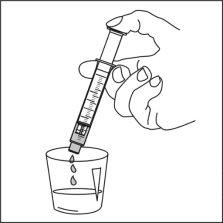
Ospolot can be taken directly from the oral syringe, or
Ospolot can be taken immediately after mixing the dose with a small amount of water or alternatively with orange juice, milk, yogurt, or wheat porridge, or
Ospolot can be administered through a feeding tube.
Instructions for use
Read these instructions carefully to know how to use this medication.
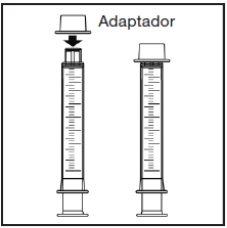
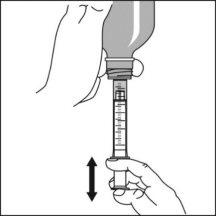
Components of the medication kit
The medication kit consists of three elements:
|
|
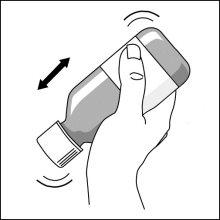
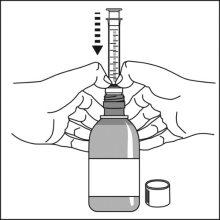
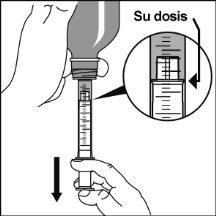
Preparation of a dose of the medication
|
Note:Leave the cap nearby to close the bottle after each use.
Note:You may not be able to insert the adapter completely, but it will be fully inserted into the bottle when you screw the cap back on. The adapter remains in the bottle after the first use. |
|
|
|
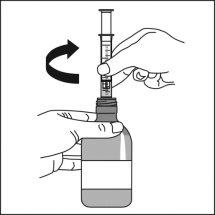
Consult your pharmacist if you have any doubts.
The adapter must always remain in the bottle. |
|
It can also be mixed with a smallamount of water, or alternatively with orange juice, milk, yogurt, or wheat porridge, just before administration. Do not take carbonated drinks or hot foods with the suspension to avoid belching or slow swallowing. Stir and take the entire mixture immediately.
|
You can take Ospolot with food, but also outside of meals. As much as possible, you should always maintain the same routine for taking Ospolot.
The oral suspension can also be administered through a feeding tube, which should be rinsed with a minimum of 15 ml of water immediately after administration. If this form of administration is used, the dose should be prepared according to the description above immediately before administration.
How long should you take Ospolot?
Antiepileptic treatment is basically long-term therapy. In each specific case, a pediatric neurologist (neuropediatrician) with experience in the treatment of epilepsy should decide how to adjust the treatment, how long it should last, and when it should be discontinued. Ospolot should not be discontinued abruptly.
If you take more Ospolot than you should
The adverse effects mentioned in the section "Possible adverse effects" may be enhanced. In case of overdose, you should consult a doctor/emergency doctor as soon as possible and, if possible, show them the medication and this prospectus or call the Toxicology Information Service, phone: 91 5620420, indicating the medication and the amount taken.
If you forget to take Ospolot
Do not take a double dose to make up for forgotten doses. Take the dose at the next scheduled time, as indicated by your doctor. You should inform the doctor in charge of your treatment.
If you interrupt treatment with Ospolot
If you wish to interrupt or stop treatment with Ospolot, consult your doctor first. Do not interrupt treatment with this medication on your own without consulting a doctor, as this could compromise the effectiveness of the treatment and cause the recurrence of epileptic seizures. The duration of treatment varies from person to person and should be determined by your doctor.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medications, this medication can cause adverse effects, although not all people experience them.
Very frequent adverse effects (may affect more than 1 in 10 people):
- stomach discomfort (e.g., nausea, vomiting)
Frequent adverse effects (may affect up to 1 in 10 people):
- difficulty breathing and even dyspnea (depending on the dose),
- chest tightness, palpitations,
- tingling in arms, legs, or face (depending on the dose),
- dizziness, headache,
- double vision,
- hiccups, weight loss or loss of appetite.
Uncommon adverse effects (may affect up to 1 in 100 people):
- hallucinations, anxiety, lack of enthusiasm,
- muscle weakness, joint pain,
- increased seizures, generalized tonic-clonic seizures.
Frequency not known (cannot be estimated from available data):
- delayed hypersensitivity reaction affecting multiple organs or systems, with fever, skin rash, inflammation of blood vessels (vasculitis), inflammation of lymph nodes, joint pain, abnormal white blood cell count, as well as hepatomegaly or splenomegaly and severe skin reactions (Stevens-Johnson syndrome, toxic epidermal necrolysis),
- acute kidney failure,
- vision loss, which can be significant, polyneuritis (simultaneous inflammation of multiple nerves),
- toxic reactions in the liver or increased liver enzyme levels,
- depressive mood/depression, personality changes, and behavioral abnormalities (e.g., aggression, irritability, mood changes), and cognitive impairment,
- diarrhea.
In a patient with long-term resistant epilepsy, administration of Ospolot caused progressive weakness of the limbs, increased salivation, difficulty speaking, and increasing somnolence until coma. These symptoms subsided a few hours after discontinuing treatment with Ospolot.
Sultiamo belongs to a group of active ingredients (carbonic anhydrase inhibitors) that can cause the formation of kidney stones and changes in blood composition (metabolic acidosis, hemodilution, and alterations in serum electrolyte concentrations, such as reduced calcium levels in blood), as well as fatigue/exhaustion.
Reporting adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is an adverse effect not listed in this prospectus. You can also report them directly through the Spanish Medication Surveillance System for Human Use: www.notificaRAM.es
By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Ospolot
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the bottle and carton after EXP. The expiration date is the last day of the month indicated.
After the first opening of the bottle, it should not be used for more than 3 months.
Do not use this medication if you observe any damage to the bottle, closure, or carton.
Medications should not be thrown down the drain. Deposit the packaging and medications you no longer need at the SIGRE collection point in the pharmacy. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Ospolot composition
The active ingredient is sultiamo.
The other ingredients are: sodium methylparahydroxybenzoate (E219), sodium propylparahydroxybenzoate (E217), sucralose (E955), sodium docusate, xanthan gum (E415), disodium dihydrogen phosphate dihydrate (E339), dipotassium phosphate (E340), strawberry flavor (contains Acacia E414), sweetness modulator flavor (with fructose, glucose, sucrose, sulfur dioxide [E220]), masking flavor contains Sucralosa E955, Maltodextrin [potato]), phosphoric acid 85% (E338) (for pH adjustment), purified water.
Appearance of the product and package contents
Ospolot oral suspension is a white suspension.
The glass bottle with child-resistant closure contains 200 ml or 250 ml of oral suspension. It is presented in a cardboard box containing a 10 ml oral syringe with graduation every 0.25 ml and an adapter.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Desitin Arzneimittel GmbH
Weg beim Jäger 214
22335 Hamburg
Germany
This medication is authorized in the Member States of the European Economic Area with the following names:
BE Ospolot 20 mg/ml oral suspension / solution buvable / Suspension zum Einnehmen
CZ Ospolot
DK Ospolot
DE Ospolot 20 mg/ml Suspension zum Einnehmen
EE Ospolot
ES Ospolot 20 mg/ml Oral Suspension
FI Ospolot 20 mg/ml Oraalisuspensio
IE Ospolot 20 mg/ml oral suspension
IT Ospolot
LU Ospolot
NL Ospolot 20 mg/ml Suspension voor oraal gebruik
NO Ospolot 20 mg/ml Mikstur, suspensjon
PL Sultiame Desitin
PT Ospolot 20 mg/ml Suspensão oral
RO Ospolot 20 mg/ml Suspensie orala
SE Ospolot 20 mg/ml Oral suspension
SK Ospolot
Date of the last revision of this prospectus:
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OSPOLOT 20 mg/ml ORAL SUSPENSIONDosage form: TABLET, 100 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: TABLET, 200 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: TABLET, 25 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription required
Online doctors for OSPOLOT 20 mg/ml ORAL SUSPENSION
Discuss questions about OSPOLOT 20 mg/ml ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions