
OPFOLDA 65 mg HARD CAPSULES

How to use OPFOLDA 65 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Opfolda 65 mg Hard Capsules
miglustat
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Opfolda and what is it used for
- What you need to know before you take Opfolda
- How to take Opfolda
- Possible side effects
- Storage of Opfolda
- Contents of the pack and other information
1. What is Opfolda and what is it used for
What is Opfolda
Opfolda is a medicine used for the treatment of late-onset Pompe disease in adults. This medicine contains the active substance “miglustat”.
What Opfolda is used for
Opfolda is always used with another medicine called “cipaglucosidase alfa”, a type of enzyme replacement therapy (ERT). Therefore, it is very important that you also read the package leaflet of cipaglucosidase alfa.
If you have any questions about these medicines, ask your doctor or pharmacist.
How Opfolda works
People with Pompe disease have low levels of an enzyme called acid alpha-glucosidase (GAA). This enzyme helps regulate the levels of glycogen (a type of carbohydrate) in the body.
In Pompe disease, large amounts of glycogen accumulate in the muscles throughout the body. This prevents the muscles from working properly, for example, the muscles that help you walk, those that facilitate breathing in the lungs, and the heart muscle.
Opfolda binds to cipaglucosidase alfa during treatment. This makes the form of cipaglucosidase alfa more stable, so it can be more easily absorbed by the muscle cells affected by Pompe disease. Once inside the cells, cipaglucosidase alfa acts like GAA, promoting the breakdown of glycogen and regulating its levels.
2. What you need to know before you take Opfolda
Do not take Opfolda
- If you are allergic to miglustat or any of the other ingredients of this medicine (listed in section 6).
- If you are allergic to alpha cypaglucosidase.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start taking Opfolda.
Pay attention to serious side effects
Opfolda is used together with cipaglucosidase alfa, a type of enzyme replacement therapy (ERT), so you should also read the package leaflet of cipaglucosidase alfa. These medicines can cause side effects that you should report to your doctor immediately, such as allergic reactions. The signs of an allergic reaction are listed in section 4 “Allergic reactions”.
These reactions can be serious and occur during or after administration of the medicine.
Tell your doctor or nurse immediately if you experience or think you may be experiencing a perfusion-associated reaction or an allergic reaction. Before taking Opfolda, tell your doctor or nurse if you have ever had a reaction of this type with another ERT.
Children and adolescents
This medicine must not be given to patients under 18 years of age, as the effects of Opfolda with cipaglucosidase alfa in this age group are unknown.
Other medicines and Opfolda
Tell your doctor or nurse if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription and herbal medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, ask your doctor or pharmacist for advice before taking this medicine.
There are no data on the use of Opfolda in combination with cipaglucosidase alfa during pregnancy. Your doctor will explain the risks and benefits of taking these medicines.
- Do not take Opfolda or receive cipaglucosidase alfa if you are pregnant. Tell your doctor immediately if you become pregnant, think you may be pregnant, or plan to become pregnant. There may be risks for the unborn baby.
- Opfolda in combination with cipaglucosidase alfa must not be used in women who are breastfeeding. A decision must be made whether to discontinue breastfeeding or discontinue the treatment.
Contraception and fertility
Women of childbearing potential must use effective contraceptive methods during treatment and for 4 weeks after stopping administration of both medicines.
Driving and using machines
Opfolda has no or negligible influence on the ability to drive and use machines. You should also read the package leaflet of cipaglucosidase alfa, as the medicine may affect the ability to drive and use machines.
3. How to take Opfolda
Follow exactly the instructions for administration of this medicine given by your doctor. If you are unsure, ask your doctor or pharmacist again.
How much Opfolda to take
- Opfolda capsules (miglustat) must be used with cipaglucosidase alfa. Also, read the package leaflet of cipaglucosidase alfa.
- If you weigh 50 kg or more, the recommended dose is 4 capsules, each containing 65 mg of miglustat.
- If you weigh between 40 kg and 50 kg, the recommended dose is 3 capsules.
How often to take Opfolda
- You will receive Opfolda and cipaglucosidase alfa every 2 weeks. Both are administered on the same day.
- Follow exactly the instructions for administration of both medicines given by your doctor, see Figure 1. This way, the treatment can work as well as possible.
Taking Opfolda with food
You must take Opfolda orally on an empty stomach.
- You will have to fast for 2 hours before and 2 hours after taking this medicine.
- During this 4-hour fasting period, you can drink water, skimmed cow's milk, and tea or coffee. Do not drink cream, whole or semi-skimmed cow's milk, plant-based milks, sugar, or sweeteners. You can drink skimmed cow's milk with tea or coffee.
- 2 hours after taking Opfolda, you can eat and drink normally again.
Figure 1. Chronological development of doses
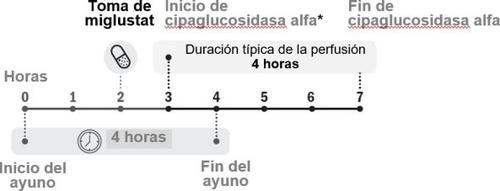
- Miglustat 65 mg hard capsule should be taken approximately 1 hour and no more than 3 hours before the start of cipaglucosidase alfa infusion.
Switching from another enzyme replacement therapy (ERT)
If you are currently receiving another ERT:
- Your doctor will tell you when to stop the other ERT before starting Opfolda.
- Tell your doctor when you received the last dose.
If you take more Opfolda than you should
Tell your doctor or go to the hospital immediately if you accidentally take more capsules than prescribed. You may be at a higher risk of side effects with this medicine (see section 4). Your doctor will provide the necessary symptomatic treatment.
If you forget to take Opfolda
If you forget to take a dose of Opfolda, talk to your doctor or nurse. Contact your doctor or nurse immediately to reschedule the administration of miglustat in combination with cipaglucosidase alfa as soon as possible.
If you stop taking Opfolda
Talk to your doctor if you want to stop taking Opfolda. Your symptoms may worsen if you stop treatment.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Opfolda is used together with cipaglucosidase alfa, and either of these medicines can cause side effects.
The following side effects may occur:
Allergic reactions
Allergic reactions can cause symptoms such as rash on any part of the body, swelling of the eyes, prolonged difficulty breathing, cough, swelling of the lips, tongue, or throat, itching of the skin, and hives.
Tell your doctor or nurse immediately if you experience or think you may be experiencing an allergic reaction. Tell your doctor or nurse if you have ever had a reaction of this type.
Very common(may affect more than 1 in 10 people)
- Headache
Common(may affect up to 1 in 10 people)
- Difficulty breathing (dyspnea)
- Sudden redness of the face, neck, or upper chest
- Increased blood pressure
- Stomach pain
- Abdominal swelling
- Flatulence or gas
- Diarrhea, loose stools
- Constipation
- Vomiting
- Fatigue
- Nausea
- Fever
- Itchy hives (urticaria)
- Itchy rash, itching (pruritus)
- Chills
- Muscle cramps, pain, or weakness
- Tremors in one or more parts of the body
- Increased sweating
- Pain
- Altered sense of taste
Uncommon(may affect up to 1 in 100 people)
- Asthma
- Allergic reaction
- Upset stomach
- Indigestion
- Pain or irritation of the throat
- Painful or abnormal contractions of the throat
- Malaise, general feeling of lethargy
- Nervousness
- Swelling of the hands, feet, ankles, legs
- Feeling of constant tiredness
- Unusual paleness of the skin
- Low blood pressure
- Decrease in platelets or a type of white blood cell (detected in blood tests)
- Joint pain
- Pain in the area between the hip and the ribs
- Muscle fatigue
- Increased muscle stiffness
- Somnolence
- Pain in one or both sides of the head, stabbing pain, aura, pain in the eyes, sensitivity to light (migraine)
- Spots on the skin
- Balance disorder
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Opfolda
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after “EXP”. The expiry date refers to the last day of the month shown.
No special storage conditions are required.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Opfolda contains
- The active substance is miglustat. Each hard capsule contains 65 mg of miglustat.
- The other ingredients are:
Contents of the capsules
Pregelatinized starch (maize)
Magnesium stearate (E470b)
Microcrystalline cellulose (E460i)
Sucralose (E955)
Colloidal silicon dioxide
Capsule shell
Gelatin
Titanium dioxide (E171)
Black iron oxide (E172)
Printing ink
Black iron oxide (E172)
Potassium hydroxide (E525)
Propylene glycol (E1520)
Strong ammonia solution (E527)
Lacquer (E904)
Appearance and packaging
Bottles of 4 and 24 capsules.
Not all pack sizes may be marketed.
Hard capsule, size 2, with grey opaque cap and white opaque body with "AT2221" printed in black on the body, containing white to off-white powder.
Marketing authorisation holder
Amicus Therapeutics Europe Limited
Block 1, Blanchardstown Corporate Park
Ballycoolin Road
Blanchardstown, Dublin
D15 AKK1
Ireland
Tel: +353 (0) 1 588 0836
Fax: +353 (0) 1 588 6851
Email: [email protected]
Manufacturer
Manufacturing Packaging Farmaca (MPF) B.V.
Neptunus 12, Heerenveen, 8448CN, Netherlands
You can request more information about this medicine from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Amicus Therapeutics Europe Limited Tél/Tel: (+32) 0800 89172 e-mail: [email protected] | Lietuva Amicus Therapeutics Europe Limited Tel: (+370) 8800 33167 El. paštas: [email protected] |
Amicus Therapeutics Europe Limited Τηλ: (+359) 00800 111 3214 | Luxembourg/Luxemburg Amicus Therapeutics Europe Limited Tél/Tel: (+352) 800 27003 e-mail: [email protected] |
Ceská republika Amicus Therapeutics Europe Limited Tel.: (+420) 800 142 207 e-mail: [email protected] | Magyarország Amicus Therapeutics Europe Limited Tel.: (+36) 06 800 21202 e-mail: [email protected] |
Danmark Amicus Therapeutics Europe Limited Tlf.: (+45) 80 253 262 e-mail: [email protected] | Malta Amicus Therapeutics Europe Limited Tel: (+356) 800 62674 e-mail: [email protected] |
Deutschland Amicus Therapeutics GmbH Tel: (+49) 0800 000 2038 e-mail: [email protected] | Nederland Amicus Therapeutics BV Tel: (+31) 0800 022 8399 e-mail: [email protected] |
Eesti Amicus Therapeutics Europe Limited Tel: (+372) 800 0111 911 e-post: [email protected] | Norge Amicus Therapeutics Europe Limited Tlf: (+47) 800 13837 e-post: [email protected] |
Ελλάδα Amicus Therapeutics Europe Limited Τηλ: (+30) 00800 126 169 e-mail: [email protected] | Österreich Amicus Therapeutics Europe Limited Tel: (+43) 0800 909 639 e-mail: [email protected] |
España Amicus Therapeutics S.L.U. Tel: (+34) 900 941 616 e-mail: [email protected] | Polska Amicus Therapeutics Europe Limited Tel.: (+48) 0080 012 15475 e-mail: [email protected] |
France Amicus Therapeutics SAS Tél: (+33) 0 800 906 788 e-mail: [email protected] | Portugal Amicus Therapeutics Europe Limited Tel: (+351) 800 812 531 e-mail: [email protected] |
Hrvatska Amicus Therapeutics Europe Limited Tel: (+358) 0800 222 452 e-pošta: [email protected] | Ireland Amicus Therapeutics Europe Limited Tel: (+353) 1800 936 230 e-mail: [email protected] |
România Amicus Therapeutics Europe Limited Tel.: (+40) 0808 034 288 e-mail: [email protected] | Slovenija Amicus Therapeutics Europe Limited Tel.: (+386) 0800 81794 e-pošta: [email protected] |
Ísland Amicus Therapeutics Europe Limited Sími: (+354) 800 7634 Netfang: [email protected] | Slovenská republika Amicus Therapeutics Europe Limited Tel: (+421) 0800 002 437 e-mail: [email protected] |
Italia Amicus Therapeutics S.r.l. Tel: (+39) 800 795 572 e-mail: [email protected] | Suomi/Finland Amicus Therapeutics Europe Limited Puh/Tel: (+358) 0800 917 780 sähköposti/e-mail: [email protected] |
Κύπρος Amicus Therapeutics Europe Limited Τηλ: (+357) 800 97595 e-mail: [email protected] | Sverige Amicus Therapeutics Europe Limited Tfn: (+46) 020 795 493 e-post: [email protected] |
Latvija Amicus Therapeutics Europe Limited Tel: (+371) 800 05391 e-pasts: [email protected] | United Kingdom (Northern Ireland) Amicus Therapeutics, UK Limited Tel: (+44) 08 0823 46864 e-mail: [email protected] |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OPFOLDA 65 mg HARD CAPSULESDosage form: CAPSULE, 100 mgActive substance: miglustatManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: CAPSULE, 100 mgActive substance: miglustatManufacturer: Dipharma Arzneimittel GmbhPrescription requiredDosage form: CAPSULE, 100 mgActive substance: miglustatManufacturer: Gen.Orph S.A.S.Prescription required
Online doctors for OPFOLDA 65 mg HARD CAPSULES
Discuss questions about OPFOLDA 65 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















