
OMVOH 300 MG CONCENTRATE FOR INFUSION SOLUTION

How to use OMVOH 300 MG CONCENTRATE FOR INFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
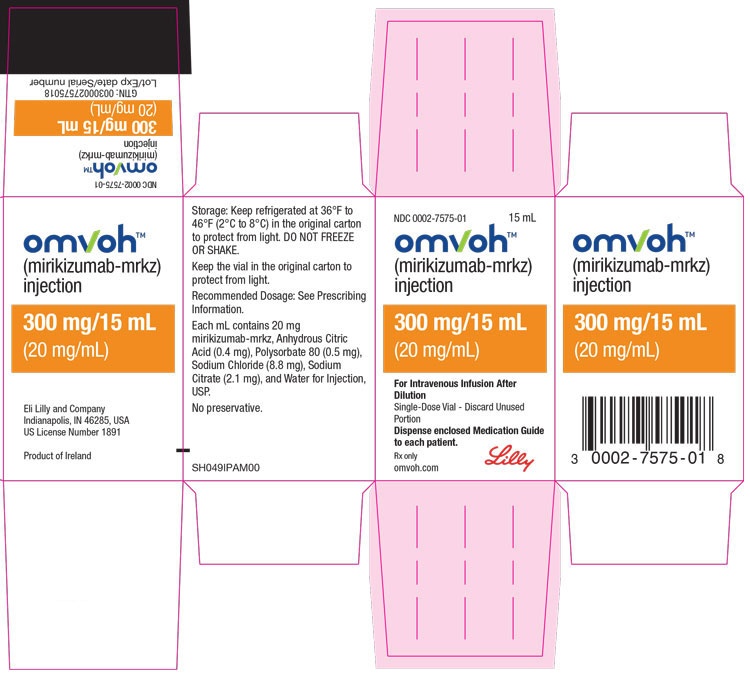
Omvoh 300 mg Concentrate for Solution for Infusion
mirikizumab
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Omvoh and what is it used for
- What you need to know before you are given Omvoh
- How Omvoh is given
- Possible side effects
- Storage of Omvoh
- Contents of the pack and other information
1. What is Omvoh and what is it used for
Omvoh is used to treat the following inflammatory bowel diseases:
- Ulcerative colitis
- Crohn's disease
Omvoh contains the active substance mirikizumab, a monoclonal antibody. Monoclonal antibodies are proteins that identify and bind to specific target proteins in the body. Omvoh works by binding to and blocking a protein in the body called IL-23 (interleukin-23), which is involved in inflammation. By blocking the action of IL-23, Omvoh reduces inflammation and other symptoms associated with ulcerative colitis and Crohn's disease.
Ulcerative colitis
Ulcerative colitis is a chronic inflammatory disease of the large intestine. If you have ulcerative colitis, you will first be given other medicines. If you do not respond well enough or cannot tolerate these medicines, you may be given Omvoh to reduce the signs and symptoms of ulcerative colitis, such as diarrhea, abdominal pain, urgency, and rectal bleeding.
Crohn's disease
Crohn's disease is a chronic inflammatory disease of the digestive tract. If you have active Crohn's disease, you will first be given other medicines. If you do not respond well enough or cannot tolerate these medicines, you may be given Omvoh to reduce the signs and symptoms of Crohn's disease, such as diarrhea, abdominal pain, fatigue, and urgency.
2. What you need to know before you are given Omvoh
Do not use Omvoh
- if you are allergic to mirikizumab or any of the other ingredients of this medicine (listed in section 6). If you think you may be allergic, consult your doctor before using Omvoh.
- if you have active important infections (active tuberculosis).
Warnings and precautions
- Consult your doctor or pharmacist before starting treatment with this medicine.
- Your doctor will check how you are before treatment.
- Make sure to inform your doctor about any disease you suffer from before treatment.
Infections
- Omvoh may potentially cause serious infections.
- Treatment with Omvoh must not be started if you have an active infection until the infection has disappeared.
- After starting treatment, tell your doctor immediately if you have any symptoms of infection, such as:
|
|
|
|
|
|
|
|
- Also, tell your doctor if you have recently been near someone who may have tuberculosis.
- Your doctor will examine you and perform a test for tuberculosis detection before using Omvoh.
- If your doctor thinks you are at risk of having active tuberculosis, you may be given medicines to treat it.
Vaccines
Your doctor will check if you need any vaccine before starting treatment. Tell your doctor, pharmacist, or nurse if you have been vaccinated recently or are going to be vaccinated. Some types of vaccines (live vaccines) must not be given while you are using Omvoh.
Allergic reactions
- Omvoh may potentially cause serious allergic reactions.
- Stop using Omvoh and seek medical attention immediately if you have any of the following symptoms of a serious allergic reaction:
|
|
|
|
|
|
Liver blood tests
Your doctor will perform a blood test before starting treatment with Omvoh and during treatment to check if your liver is working normally. If the blood tests are abnormal, your doctor may stop treatment with Omvoh and perform additional tests on your liver to determine the cause.
Children and adolescents
Omvoh is not recommended for use in children and adolescents under 18 years of age, as it has not been studied in this age group.
Other medicines and Omvoh
Tell your doctor, pharmacist, or nurse
- if you are using, have recently used, or might use any other medicine.
- if you have been vaccinated recently or are going to be vaccinated. Some types of vaccines (live vaccines) must not be given while you are using Omvoh.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. It is preferable to avoid the use of Omvoh during pregnancy. The effects of Omvoh in pregnant women are not known. If you are a woman of childbearing age, you are advised to avoid becoming pregnant and to use an adequate contraceptive method while you are using Omvoh and for at least 10 weeks after the last dose of Omvoh.
If you are breastfeeding, or plan to breastfeed, consult your doctor before using this medicine.
Driving and using machines
Omvoh is unlikely to affect your ability to drive or use machines.
Omvoh contains sodium
This medicine contains 60 mg of sodium (main component of cooking/table salt) in each 300 mg dose for the treatment of ulcerative colitis. This is equivalent to 3% of the maximum recommended daily intake of sodium for an adult.
This medicine contains 180 mg of sodium (main component of cooking/table salt) in each 900 mg dose for the treatment of Crohn's disease. This is equivalent to 9% of the maximum recommended daily intake of sodium for an adult.
Before Omvoh is given to you, it is mixed with a solution that may contain sodium. Consult your doctor if you are on a low-salt diet.
Omvoh contains polysorbate
This medicine contains 0.5 mg/ml of polysorbate 80 in each vial, equivalent to 7.5 mg for the induction dose to treat ulcerative colitis and equivalent to 22.5 mg for the induction dose to treat Crohn's disease. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergy.
3. How Omvoh is given
Omvoh must be used under the guidance and supervision of a doctor with experience in the diagnosis and treatment of ulcerative colitis and Crohn's disease.
How much Omvoh is given and for how long
Your doctor will decide how much Omvoh you need and the duration of treatment. Omvoh is for long-term treatment. Your doctor or nurse will monitor your condition regularly to check that the treatment is having the desired effect.
Ulcerative colitis
- Start of treatment: the first dose of Omvoh is 300 mg and your doctor will give it to you by intravenous infusion (drip into a vein in your arm) over at least 30 minutes. After the first dose, you will receive another dose of 300 mg of Omvoh 4 weeks later and again 4 weeks after that.
If you do not have an adequate therapeutic response after these 3 infusions, your doctor may consider continuing with intravenous infusions at weeks 12, 16, and 20.
- Maintenance treatment: 4 weeks after the last intravenous infusion, you will be given a maintenance dose of 200 mg of Omvoh by injection under the skin (“subcutaneously”) and then every 4 weeks. The maintenance dose of 200 mg will be given as 2 injections of 100 mg of Omvoh each.
If you lose response after receiving the maintenance dose of Omvoh, your doctor may decide to give you 3 doses of Omvoh by intravenous infusions.
Your doctor or nurse will tell you when to switch to subcutaneous injections.
During maintenance treatment, you and your doctor or nurse must decide if you should inject Omvoh yourself after receiving training in the subcutaneous injection technique. It is important that you do not try to inject yourself until your doctor or nurse has taught you. Your doctor or nurse will provide the necessary training.
Crohn's disease
- Start of treatment: the first dose of Omvoh is 900 mg (3 vials with 300 mg each) and your doctor will give it to you by intravenous infusion (drip into a vein in your arm) over at least 90 minutes. After the first dose, you will receive another dose of 900 mg of Omvoh 4 weeks later and again 4 weeks after that.
- Maintenance treatment: 4 weeks after the last intravenous infusion, you will be given a maintenance dose of 300 mg of Omvoh by injection under the skin (“subcutaneously”) and then every 4 weeks. The maintenance dose of 300 mg will be given with a 100 mg pre-filled syringe or pen and a 200 mg pre-filled syringe or pen. The injections can be given in any order.
The 200 mg pre-filled syringe or pen is only for the treatment of Crohn's disease.
Your doctor or nurse will tell you when to switch to subcutaneous injections.
During maintenance treatment, you and your doctor or nurse must decide if you should inject Omvoh yourself after receiving training in the subcutaneous injection technique. It is important that you do not try to inject yourself until your doctor or nurse has taught you. Your doctor or nurse will provide the necessary training.
If you are given more Omvoh than you should
If you have been given more Omvoh than you should or the dose has been given earlier than prescribed, tell your doctor.
If you miss a dose of Omvoh
If you miss a dose of Omvoh, consult your doctor.
If you stop treatment with Omvoh
Do not stop treatment with Omvoh without consulting your doctor first. If you stop treatment, the symptoms of your disease may come back.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common(may affect more than 1 in 10 people)
- Reactions at the injection site (e.g. redness of the skin, pain)
Common(may affect up to 1 in 10 people)
- Upper respiratory tract infections (infections of the nose and throat)
- Joint pain
- Headache
- Rash
Uncommon(may affect up to 1 in 100 people)
- Herpes
- Infusion-related allergic reaction (e.g. itching, hives)
- Increased liver enzyme levels in blood
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that the side effects are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Omvoh
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial label and on the outer packaging after “EXP”. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C to 8°C). Do not freeze.
Keep the vial in the outer packaging to protect it from light.
Do not use this medicine if you notice that the vial is damaged, or the medicine is cloudy, significantly brown, or has particles.
This medicine is for single use only.
Medicines should not be disposed of via wastewater or household waste. Ask your doctor, nurse, or pharmacist how to dispose of medicines no longer required. This will help protect the environment.
Diluted solution
It is recommended to start the infusion immediately after dilution. If not used immediately, the diluted solution prepared with sodium chloride 9 mg/ml (0.9%) injection solution can be stored refrigerated (2°C to 8°C) for no more than 96 hours or at room temperature not exceeding 25°C for no more than 10 hours (the total time should not exceed 96 hours) from the time of vial puncture. The infusion solution prepared with glucose 5% must be used within 48 hours, of which no more than 5 hours can be at non-refrigerated temperature above 25°C, from the time of vial puncture.
From a microbiological point of view, the product should be used immediately. If not used immediately, the in-use storage times and conditions of the reconstituted product are the responsibility of the user and would normally not be longer than 24 hours at a temperature of 2°C to 8°C, unless the dilution has been made in controlled and validated aseptic conditions.
Do not dilute the infusion solution with other solutions or infuse in combination with other electrolytes or medicines.
Keep the diluted solution away from heat or direct light.
Do not freeze the diluted solution.
6. Container contents and additional information
Omvoh composition
- The active ingredient is mirikizumab.
Each vial contains 300 mg of mirikizumab in 15 ml (20 mg/ml).
- The other components are sodium citrate dihydrate (E 331); citric acid, anhydrous (E 330); sodium chloride; polysorbate 80 (E 433); water for injectable preparations.
Product appearance and container contents
Omvoh is a solution in a transparent glass vial. Its color may vary from colorless to slightly yellow.
Package sizes of 1 vial and 3 vials. Only some package sizes may be marketed.
Marketing authorization holder
Eli Lilly Nederland B.V.,
Papendorpseweg 83
3528 BJ Utrecht
Netherlands
Manufacturer
Lilly France S.A.S.
Rue du Colonel Lilly
67640 Fegersheim
France
Lilly S.A.
Avda. de la Industria Nº 30
28108 Alcobendas, Madrid
Spain
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium/België/Belgien Eli Lilly Benelux S.A./N.V. Tel: + 32-(0)2 548 84 84 | Lithuania Eli Lilly Lietuva Tel: +370 (5) 2649600 |
| Luxembourg/Luxemburg Eli Lilly Benelux S.A./N.V. Tél/Tel: + 32-(0)2 548 84 84 |
Czech Republic ELI LILLY CR, s.r.o. Tel: + 420 234 664 111 | Hungary Lilly Hungária Kft. Tel: + 36 1 328 5100 |
Denmark Eli Lilly Danmark A/S Tlf.: +45 45 26 60 00 | Malta Charles de Giorgio Ltd. Tel: + 356 25600 500 |
Germany Lilly Deutschland GmbH Tel. + 49-(0) 6172 273 2222 | Netherlands Eli Lilly Nederland B.V. Tel: + 31-(0) 30 60 25 800 |
Estonia Eli Lilly Nederland B.V. Tel: +372 6 817 280 | Norway Eli Lilly Norge A.S. Tlf: + 47 22 88 18 00 |
Greece ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε. Τηλ: +30 210 629 4600 | Austria Eli Lilly Ges.m.b.H. Tel: + 43-(0) 1 711 780 |
Spain Lilly S.A. Tel: + 34-91 663 50 00 | Poland Eli Lilly Polska Sp. z o.o. Tel: +48 22 440 33 00 |
France Lilly France Tél: +33-(0) 1 55 49 34 34 | Portugal Lilly Portugal Produtos Farmacêuticos, Lda Tel: + 351-21-4126600 |
Croatia Eli Lilly Hrvatska d.o.o. Tel: +385 1 2350 999 | Romania Eli Lilly România S.R.L. Tel: + 40 21 4023000 |
Ireland Eli Lilly and Company (Ireland) Limited Tel: + 353-(0) 1 661 4377 | Slovenia Eli Lilly farmacevtska družba, d.o.o. Tel: +386 (0)1 580 00 10 |
Iceland Icepharma hf. Sími + 354 540 8000 | Slovakia Eli Lilly Slovakia, s.r.o. Tel: + 421 220 663 111 |
Italy Eli Lilly Italia S.p.A. Tel: + 39- 055 42571 | Finland Oy Eli Lilly Finland Ab Puh/Tel: + 358-(0) 9 85 45 250 |
Cyprus Phadisco Ltd Τηλ: +357 22 715000 | Sweden Eli Lilly Sweden AB Tel: + 46-(0) 8 7378800 |
Latvia Eli Lilly (Suisse) S.A. Parstavnieciba Latvija Tel: +371 67364000 |
Date of last revision of this project:
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (https://www.aemps.gob.es/).
-----------------------------------------------------------------------------------------------------------
Omvoh 300 mg concentrate for solution for infusion
mirikizumab
This information is intended only for healthcare professionals:
Omvoh should not be used if it has been frozen.
Disposal of unused medicinal product and all materials that have come into contact with it should be done in accordance with local regulations.
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the administered medicinal product should be clearly recorded.
Dilution prior to intravenous infusion
- Each vial is for single use.
- Prepare the infusion solution using an aseptic technique to ensure the sterility of the prepared solution.
- Inspect the vial contents. The concentrate should be transparent, colorless to slightly yellow, and free of visible particles. Otherwise, it should be discarded.
- Prepare the infusion bag for the treatment of ulcerative colitis or Crohn's disease as specified below. Note that there are unique instructions and volumes specified for each indication.
Ulcerative colitis: one vial of 15 ml (300 mg)
Withdraw 15 ml from the mirikizumab vial (300 mg) using a suitable-sized needle (18-21 G recommended) and transfer to the infusion bag. If administered for ulcerative colitis, the concentrate should be diluted only in infusion bags (bag size ranges from 50 - 250 ml) containing a 9 mg/ml (0.9%) sodium chloride injection solution or a 5% glucose injection solution. The final concentration after dilution is approximately 1.1 mg/ml to approximately 4.6 mg/ml.
Crohn's disease: three vials of 15 ml; total volume = 45 ml (900 mg)
First, withdraw and discard 45 ml of diluent from the infusion bag. Then, withdraw 15 ml from each of the three mirikizumab vials (900 mg) and transfer to the infusion bag, using a syringe and a suitable-sized needle (18-21 G recommended). If administered for Crohn's disease, the concentrate should be diluted only in infusion bags (bag size ranges from 100 - 250 ml) containing a 9 mg/ml (0.9%) sodium chloride injection solution or a 5% glucose injection solution.
The final concentration after dilution is approximately 3.6 mg/ml to approximately 9 mg/ml.
- Gently invert the infusion bag to mix. Do not shake the prepared bag.
Administration of the diluted solution
- The intravenous administration equipment (infusion line) should be connected to the prepared infusion bag and the line should be purged.
For ulcerative colitis, the infusion should be administered over at least 30 minutes.
For Crohn's disease, the infusion should be administered over at least 90 minutes.
- At the end of the infusion, to ensure that a full dose is administered, the infusion line should be flushed with a 9 mg/ml (0.9%) sodium chloride solution or a 5% glucose injection solution. The flushing should be administered at the same rate as used for the administration of Omvoh. The time required to flush the Omvoh solution from the infusion line is added to the minimum infusion time of 30 minutes (ulcerative colitis) or 90 minutes (Crohn's disease).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OMVOH 300 MG CONCENTRATE FOR INFUSION SOLUTIONDosage form: INJECTABLE, 100 mgActive substance: mirikizumabManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE, 100 mgActive substance: mirikizumabManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 130 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for OMVOH 300 MG CONCENTRATE FOR INFUSION SOLUTION
Discuss questions about OMVOH 300 MG CONCENTRATE FOR INFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







