OMVOH 100 mg/200 mg injectable solution in prefilled pen


How to use OMVOH 100 mg/200 mg injectable solution in prefilled pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Omvoh 100 mg solution for injection in pre-filled pen
Omvoh 200 mg solution for injection in pre-filled pen
mirikizumab
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Omvoh and what is it used for
- What you need to know before you use Omvoh
- How to use Omvoh
- Possible side effects
- Storage of Omvoh
- Contents of the pack and further information
1. What is Omvoh and what is it used for
Omvoh contains the active substance mirikizumab, a monoclonal antibody.
Monoclonal antibodies are proteins that identify and bind specifically to certain target proteins in the body. Omvoh works by binding to and blocking a protein in the body called IL-23 (interleukin-23), which is involved in inflammation. By blocking the action of IL-23, Omvoh reduces inflammation and other symptoms associated with Crohn's disease.
Crohn's Disease
Crohn's disease is a chronic inflammatory disease of the digestive tract. If you have active Crohn's disease, you will first be given other medicines. If you do not respond well enough or cannot tolerate these medicines, you may be given Omvoh to reduce the signs and symptoms of Crohn's disease, such as diarrhea, abdominal pain, fatigue, and urgency.
2. What you need to know before you use Omvoh
Do not use Omvoh
- if you are allergic to mirikizumab or any of the other ingredients of this medicine (listed in section 6). If you think you may be allergic, consult your doctor before using Omvoh.
- if you have active important infections (active tuberculosis).
Warnings and precautions
- Talk to your doctor or pharmacist before starting this medicine.
- Your doctor will check how you are before treatment.
- Make sure to tell your doctor about any illness you have before treatment.
Infections
- Omvoh may potentially cause serious infections. Do not start treatment with Omvoh if you have an active infection until the infection has disappeared.
- After starting treatment, tell your doctor immediately if you have any symptoms of infection, such as:
|
|
|
|
|
|
|
|
- Also, tell your doctor if you have recently been near someone who may have tuberculosis.
- Your doctor will examine you and perform a test to detect tuberculosis before using Omvoh.
- If your doctor thinks you are at risk of having active tuberculosis, you may be given medicines to treat it.
Vaccines
Your doctor will check if you need any vaccine before starting treatment. Tell your doctor, pharmacist, or nurse if you have been vaccinated recently or are going to be vaccinated. Some types of vaccines (live vaccines) should not be given while using Omvoh.
Allergic reactions
- Omvoh may potentially cause serious allergic reactions.
- Stop using Omvoh and seek medical attention immediately if you have any of the following symptoms of a serious allergic reaction:
|
|
|
tongue, or throat, difficulty breathing |
|
chest. |
Liver blood tests
Your doctor will perform a blood test before starting treatment with Omvoh and during treatment to check if your liver is working normally. If the blood tests are abnormal, your doctor may stop treatment with Omvoh and perform additional tests on your liver to determine the cause.
Children and adolescents
Omvoh is not recommended for use in children and adolescents under 18 years of age, as it has not been studied in this age group.
Other medicines and Omvoh
Tell your doctor, pharmacist, or nurse
- if you are using, have recently used, or might use any other medicines.
- if you have been vaccinated recently or are going to be vaccinated. Some types of vaccines (live vaccines) should not be given while using Omvoh.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. It is preferable to avoid the use of Omvoh during pregnancy. The effects of Omvoh in pregnant women are not known. If you are a woman of childbearing potential, you are advised to avoid becoming pregnant and to use an adequate method of contraception while using Omvoh and for at least 10 weeks after the last dose of Omvoh.
If you are breastfeeding, or planning to breastfeed, ask your doctor for advice before taking this medicine.
Driving and using machines
Omvoh is unlikely to affect your ability to drive or use machines.
Omvoh contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
Omvoh contains polysorbate
This medicine contains 0.3 mg/ml of polysorbate 80 in each pre-filled pen, equivalent to 0.9 mg for the maintenance dose for treating Crohn's disease. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergy.
3. How to use Omvoh
Follow exactly the administration instructions of this medicine given by your doctor or nurse. If you are unsure, consult your doctor, nurse, or pharmacist again.
How much Omvoh is administered and for how long
Your doctor will decide the amount of Omvoh you need and the duration of treatment. Omvoh is for long-term treatment. Your doctor or nurse will monitor your condition periodically to check that the treatment is having the desired effect.
Crohn's Disease
- Start of treatment: the first dose of Omvoh is 900 mg (3 vials of 300 mg each) and your doctor will administer it to you by intravenous infusion (drip into a vein in your arm) for at least 90 minutes. After the first dose, you will receive another dose of 900 mg of Omvoh 4 weeks later and again 4 weeks later.
- Maintenance treatment: 4 weeks after the last intravenous infusion, you will receive a maintenance dose of 300 mg of Omvoh by injection under the skin ("subcutaneously") and then every 4 weeks. The maintenance dose of 300 mg will be administered by 2 injections: one containing 100 mg (1 ml) of Omvoh and another containing 200 mg (2 ml) of Omvoh. The injections can be administered in any order. The 200 mg pre-filled pen is only for the treatment of Crohn's disease.
Your doctor or nurse will tell you when to switch to subcutaneous injections.
During maintenance treatment, you and your doctor or nurse should decide if you should inject Omvoh yourself after receiving training in the subcutaneous injection technique. It is important that you do not try to inject yourself until your doctor or nurse has taught you. Your doctor or nurse will provide the necessary training. A caregiver can also administer the Omvoh injection after proper training.
Use a reminder method, such as notes on a calendar or diary, to help you remember when to administer your next dose to avoid missing or repeating a dose.
If you receive more Omvoh than you should
If you have received more Omvoh than you should or the dose has been administered before it was due, tell your doctor.
If you forget to use Omvoh
If you have forgotten to inject a dose of Omvoh, inject it as soon as possible. Then, resume administration every 4 weeks.
If you stop treatment with Omvoh
Do not stop treatment with Omvoh without consulting your doctor first. If you stop treatment, the symptoms of your disease may return.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common(may affect more than 1 in 10 people):
- Reactions at the injection site (e.g., redness of the skin, pain)
Common(may affect up to 1 in 10 people):
- Upper respiratory tract infections (infections of the nose and throat)
- Joint pain
- Headache
- Rash
Uncommon(may affect up to 1 in 100 people):
- Herpes
- Infusion-related allergic reaction (e.g., itching, hives)
- Increased liver enzyme levels in blood.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Omvoh
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and on the outer packaging after "EXP". The expiry date is the last day of the month stated.
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
Do notheat the pre-filled pens in the microwave, do not soak them in hot water, and do not expose them to direct sunlight.
Do notshake the pre-filled pen.
Store in the original packaging to protect from light.
Omvoh can be stored without refrigeration for up to 2 weeks at a temperature not above 30 °C.
If these conditions are exceeded, Omvoh should be discarded.
Do not use this medicine if you notice that the pre-filled pen is damaged or the medicine is cloudy, significantly brown, or has particles.
This medicine is for single use only.
Medicines should not be disposed of via wastewater or household waste. Ask your doctor, nurse, or pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Omvoh Composition
- The active ingredient is mirikizumab.
A pre-filled pen contains 100 mg of mirikizumab in 1 ml of solution, and a pre-filled pen contains 200 mg of mirikizumab in 2 ml of solution.
- The other components are histidine; histidine hydrochloride; sodium chloride; mannitol (E 421); polysorbate 80 (E 433); water for injectable preparations.
Product Appearance and Package Contents
Omvoh is a solution in a transparent glass cartridge inserted into a disposable pen, for single use. Its color may vary from colorless to slightly yellow.
Omvoh is presented in packages containing 2 pre-filled pens and in multiple packages of 3 cartons, each containing 2 pre-filled pens.
Only some package sizes may be marketed.
Marketing Authorization Holder
Eli Lilly Nederland
B.V., Papendorpseweg 83
3528 BJ Utrecht
Netherlands
Manufacturer
Lilly France S.A.S.
Rue du Colonel Lilly
67640 Fegersheim
France
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/België/Belgien Eli Lilly Benelux S.A./N.V. Tel: + 32-(0)2 548 84 84 | Lithuania Eli Lilly Lietuva Tel: +370 (5) 2649600 |
| Luxembourg/Luxemburg Eli Lilly Benelux S.A./N.V. Tel: + 32-(0)2 548 84 84 |
Czech Republic ELI LILLY CR, s.r.o. Tel: + 420 234 664 111 | Hungary Lilly Hungária Kft. Tel: + 36 1 328 5100 |
Denmark Eli Lilly Danmark A/S Tel: +45 45 26 60 00 | Malta Charles de Giorgio Ltd. Tel: + 356 25600 500 |
Germany Lilly Deutschland GmbH Tel: + 49-(0) 6172 273 2222 | Netherlands Eli Lilly Nederland B.V. Tel: + 31-(0) 30 60 25 800 |
Estonia Eli Lilly Nederland B.V. Tel: +372 6 817 280 | Norway Eli Lilly Norge A.S. Tel: + 47 22 88 18 00 |
Greece ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε. Tel: +30 210 629 4600 | Austria Eli Lilly Ges.m.b.H. Tel: + 43-(0) 1 711 780 |
Spain Lilly S.A. Tel: + 34-91 663 50 00 | Poland Eli Lilly Polska Sp. z o.o. Tel: +48 22 440 33 00 |
France Lilly France Tel: +33-(0) 1 55 49 34 34 | Portugal Lilly Portugal Produtos Farmacêuticos, Lda Tel: + 351-21-4126600 |
Croatia Eli Lilly Hrvatska d.o.o. Tel: +385 1 2350 999 | Romania Eli Lilly România S.R.L. Tel: + 40 21 4023000 |
Ireland Eli Lilly and Company (Ireland) Limited Tel: + 353-(0) 1 661 4377 | Slovenia Eli Lilly farmacevtska družba, d.o.o. Tel: +386 (0)1 580 00 10 |
Iceland Icepharma hf. Tel: + 354 540 8000 | Slovakia Eli Lilly Slovakia, s.r.o. Tel: + 421 220 663 111 |
Italy Eli Lilly Italia S.p.A. Tel: + 39- 055 42571 | Finland Oy Eli Lilly Finland Ab Tel: + 358-(0) 9 85 45 250 |
Cyprus Phadisco Ltd Tel: +357 22 715000 | Sweden Eli Lilly Sweden AB Tel: + 46-(0) 8 7378800 |
Latvia Eli Lilly (Suisse) S.A. Parstavnieciba Latvija Tel: +371 67364000 |
Date of Last Revision of this Prospectus:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (https://www.aemps.gob.es/).
Instructions for Use
Omvoh 100 mg Solution for Injection in Pre-filled Pen
Omvoh 200 mg Solution for Injection in Pre-filled Pen
mirikizumab
2 pre-filled pens: 1 pen of 100 mg and 1 pen of 200 mg

Read this before injecting Omvoh. Follow all the step-by-step instructions.
|
Also, note:
- Your healthcare professional should teach you how to prepare and inject Omvoh using the pen. Do notinject yourself or inject another person until you have been shown how to inject Omvoh.
- Each Omvoh pen is for single use. Do not share or reuse your pen. You may transmit or be transmitted an infection.
- Your healthcare professional may help you decide where on your body to inject your dose. You can also read in these instructions the section “Choose your injection site” to help you choose which area may be best for you.
- If you have vision or hearing problems, do not use the Omvoh pen without the help of a caregiver.
- Keep the instructions for use and re-read them if you need to.
Before using the Omvoh pens, carefully read and follow all the step-by-step instructions.
2 pens = complete dose of 300 mg
After your first injection, choosea new injection site at least 5 centimeters away and clean it.
With your second pen, repeat steps 1-3immediately after your first injection.
You must inject 2 pens to complete your full dose of 300 mg
Parts of the Omvoh Pen
Inject both pens in any order for a complete dose of 300 mg. The 200 mg pen is larger than the 100 mg pen.
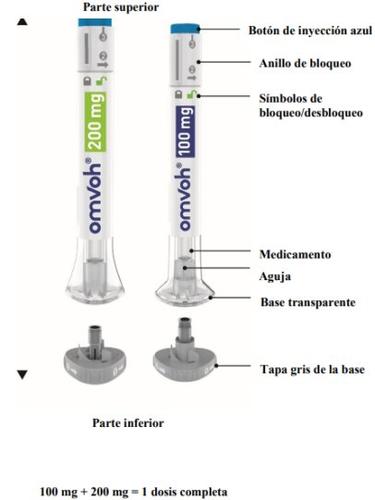
IMPORTANT:
|
Preparation to Inject Omvoh
Remove the pens from the refrigerator | Remove 2 Omvoh pens from the refrigerator. Leave the gray caps on the base until you are ready to inject. Leave the pens at room temperature for 45 minutes before injecting. Do notheat the pens in the microwave, do not wet them with hot water, or expose them to direct sunlight. Do notuse the pens if the medicine is frozen. Do notshake. |
Gather the necessary items | Necessary items:
|
Inspect the pens and medicine | Make sure you have the correct medicine. The medicine inside should be transparent. The color may vary from colorless to slightly yellow. |
Expiry Date
| Do notuse the pens, and dispose of them according to your healthcare professional's instructions if:
|
Prepare to inject | Wash your hands with water and soap before injecting Omvoh. |
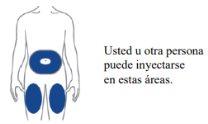
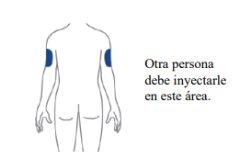
Choose your injection site
| Your healthcare professional may help you choose the best injection site for you.
example, if your first injection was in your abdomen, your second injection – to complete a full dose – could be in another area of your abdomen.
Clean the injection site with an alcohol wipe. Let the area where you will inject dry before injecting your medicine. |
Inject Omvoh
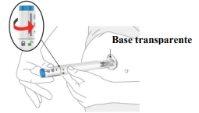
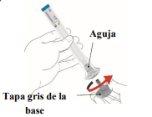
1. Remove the cap
locked. Leave the gray cap on the base until you are ready to inject.
throw it away in your household trash.
base – this could damage the needle.
|
|
2. Place and unlock
base flat and firmly against your skin
lock ring to the unlockposition. |
|
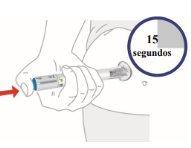
3. Hold for 15 seconds
You will hear a loud click (start of injection).
base firmly against your skin.You will hear a second loud click about 15 seconds after the first one (complete injection). |
|
Remove the pen from your skin.
Do notrub the injection site |
|
2 injections are needed for a complete dose. Inject one pen followed immediately by the other pen. |
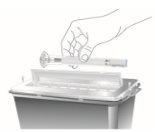
Dispose of Omvoh Pens
Dispose of used pens |
|
after use. Do not throw the pen away in your household trash. |
- If you do not have a container for disposing of sharps, you can use a household container that is:
- made of heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant,
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you should follow your community guidelines for the proper disposal of the container. There may be local regulations regarding the disposal of needles and pens.
- Do not recycle your used sharps disposal container.
- For more information on how to dispose of the container properly, ask your healthcare professional about the options available in your area.
Frequently Asked Questions
- What if I let my pens come to room temperature for more than 45 minutes before injection?
- Your pen can remain at room temperature up to 30°C for a maximum of 2 weeks.
- What if I see air bubbles in the pen?
- It is normal to have air bubbles in the pen. They will not harm you or affect your dose.
- What if there is a drop of liquid on the needle tip when I remove the gray cap from the base?
- A drop of liquid on the needle tip is normal. It will not harm you or affect your dose.
- What if I unlock the pen and press the blue injection button until the injection is complete?
- Do not remove the gray cap from the base. Do not use the pen. Consult your doctor or pharmacist to obtain a new one.
- Do I need to hold the blue injection button until the injection is complete?
- You do not need to hold the blue injection button, but it can help you keep the pen stable and firm on your skin.
- What if the needle does not retract after my injection?
- Do not touch the needle or put the gray cap back on the base. Store the pen in a safe place to avoid accidental punctures and contact your doctor, pharmacist, or nurse.
- What if there is a drop of liquid or blood on my skin after the injection?
- This is normal. Press a cotton ball or gauze over the injection site. Do notrub the injection site.
- What if I hear more than 2 clicks during my injection - 2 loud clicks and one soft click? Did I receive my complete injection?
- Some patients may hear a soft click just before the second loud click. This is the normal operation of the pen. Do not remove the pen from your skin until you hear the second loud click.
- How do I know if my injection is complete?
- After pressing the blue injection button, you will hear 2 loud clicks. The second loud click indicates that your injection is complete. You will also see the gray plunger in the top of the transparent base.
To learn more about your medicine, read the complete Omvoh prospectus inside this package.
Last Revision
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OMVOH 100 mg/200 mg injectable solution in prefilled penDosage form: INJECTABLE, 100 mgActive substance: mirikizumabManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 300 mgActive substance: mirikizumabManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 130 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for OMVOH 100 mg/200 mg injectable solution in prefilled pen
Discuss questions about OMVOH 100 mg/200 mg injectable solution in prefilled pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







 Make sure the pen is
Make sure the pen is
 Hold the base against your skin and turn the
Hold the base against your skin and turn the