
How to use Olfen Path
Patient Information Leaflet: User Information
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Olfen Patch (Olfen)
140 mg, medicinal patch
Diclofenac sodium
Olfen Patch and Olfen are different trade names for the same medicine.
Before using the medicine, carefully read the contents of the leaflet, as it contains
important information for the patient.
This medicine should always be used exactly as described in the patient information leaflet or as advised by
your doctor or pharmacist.
- You should keep this leaflet, so you can read it again if you need to
- You should consult your pharmacist if you need advice or additional information.
- If you experience any side effects, including any possible side effects not listed in the leaflet, you should tell your doctor or pharmacist. See section 4. If you do not feel better or feel worse after 3 days, you should contact your doctor.
Table of Contents of the Leaflet:
- 1. What is Olfen Patch and what is it used for
- 2. Important information before using Olfen Patch
- 3. How to use Olfen Patch
- 4. Possible side effects
- 5. How to store Olfen Patch
- 6. Contents of the packaging and other information
1. What is Olfen Patch and what is it used for
Olfen Patch is a pain-relieving medicine. It belongs to a group of non-steroidal anti-inflammatory drugs (NSAIDs).
Olfen Patch is used locally for short-term therapy to relieve pain associated with
sudden strains, sprains, or bruises in the arms and legs resulting from blunt injuries.
2. Important information before using Olfen Patch
When not to use Olfen Patch
- if you are allergic to diclofenac, propylene glycol, butylhydroxytoluene, or any of the other ingredients of this medicine (listed in section 6),
- if you are allergic to any other non-steroidal anti-inflammatory drug (NSAID, e.g., acetylsalicylic acid, ibuprofen),
- if you have had asthma attacks, skin rash, or nasal congestion and irritation when taking acetylsalicylic acid or another NSAID,
- if you have an active stomach or duodenal ulcer,
- on wounds or open skin lesions (e.g., abrasions, cuts, burns), in case of skin infections or eczema,
- during the last three months of pregnancy.
Warnings and precautions
Before starting to use Olfen Patch, you should discuss with your doctor or pharmacist:
- if you have or have had asthma or allergies, you may experience bronchospasm, which is characterized by difficulty breathing,
- if you have kidney, heart, or liver disorders or have a tendency to gastrointestinal ulcers, enteritis, or bleeding.
Side effects can be reduced by using the smallest possible doses of the medicine for the shortest possible time.
Important warnings:
- If symptoms do not improve after 3 days, you should contact your doctor.
- Do not apply the medicinal patch to the eyes or mucous membranes. Do not allow contact with the eyes and mucous membranes.
The patch should only be applied to undamaged skin. It should not be worn during bathing.
You should avoid exposing the treated areas, after removing the medicinal patch, to direct sunlight and sunlamps for about one day after removing the Olfen Patch, to reduce the risk of photosensitivity.
You should not use diclofenac-containing medicines or NSAIDs locally or systemically at the same time.
Olfen Patch and other medicines
You should tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to use. If Olfen Patch is used correctly, then only a very small amount of diclofenac penetrates the body, so interactions possible with oral administration of the medicine should not occur.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Pregnancy
In the first six months of pregnancy, Olfen Patch should only be used after consulting a doctor.
During the last three months of pregnancy, you should not use Olfen Patch due to the increased risk of complications for the mother and child (see "When not to use Olfen Patch").
Fertility
Olfen Patch should not be used in women who plan to become pregnant or have difficulty becoming pregnant, or are undergoing tests to determine the cause of infertility.
Breastfeeding
Before using any medicine, you should consult your doctor or pharmacist.
Only small amounts of diclofenac pass into breast milk. In the case of short-term use of Olfen Patch, it is not necessary to interrupt breastfeeding, as no adverse effects have been observed in the infant.
However, Olfen Patch should not be applied near the breasts.
Driving and using machines
Olfen Patch does not affect or slightly affects the ability to drive and use machines.
Olfen Patch contains propylene glycol and butylhydroxytoluene.
The medicine contains 1400 mg of propylene glycol in each patch. Propylene glycol may cause skin irritation.
Butylhydroxytoluene may cause local skin reactions (e.g., contact dermatitis) or eye and mucous membrane irritation.
3. How to use Olfen Patch
This medicine should always be used exactly as advised by your doctor. If you are unsure, you should consult your doctor or pharmacist.
Usual dose:
Apply the medicinal patch to the painful area twice a day, in the morning and evening. You can use no more than two medicinal patches per day, even if the injuries occur in several places. You should only use it on one painful area at a time.
Olfen Patch should only be applied to undamaged, unaltered skin.
Elderly patients
Elderly patients should use Olfen Patch with special caution, as they are more likely to experience side effects.
Patients with kidney, heart, or liver disorders
In patients with kidney, heart, or liver disorders, Olfen Patch should be used with special caution.
Children and adolescents under 16 years of age
There is insufficient data on the efficacy and safety of use in children and adolescents under 16 years of age.
Method of administration:
For skin use only. Do not swallow!
- 1. Open the packaging containing the medicinal patch by cutting it along the marked line.
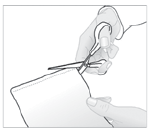
- 2. Remove the patch and close the packaging tightly, carefully pressing the edges.
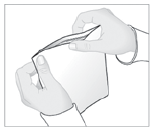
- 3. Remove the protective layer from the adhesive surface of the patch.
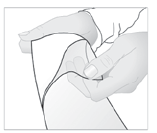
- 4. Apply the patch to the painful area.
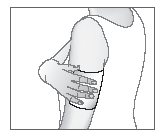
If necessary, a knitted bandage can be used to secure the patch in the desired position.
Do not use the medicinal patch under a non-breathable (occlusive) dressing.
Do not divide the patch into parts.
Used patches should be folded in half, with the adhesive side inward.
Duration of treatment
Due to limited data, only short-term therapy is recommended.
You should not use Olfen Patch for more than 3 days without consulting a doctor.
The therapeutic benefits of using the patch for more than 7 days have not been confirmed.
In the case of adolescents aged 16 and adults, if it is necessary to use the medicine for more than 7 days to relieve pain or if symptoms worsen, parents or patients should consult a doctor.
Using more than the recommended dose of Olfen Patch
In case of severe side effects due to accidental overdose (e.g., by children) or incorrect use of Olfen Patch, you should contact a doctor. Based on the assessment of the degree of poisoning, the doctor will decide what remedial actions to take.
Missing a dose of Olfen Patch
You should not use a double dose of the medicine to make up for a missed dose.
If you have any further doubts about using the medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Olfen Patch can cause side effects, although not everybody gets them.
You should stop using the patch and immediately consult a doctor if you notice any of the following symptoms:
sudden itchy rash (hives), swelling of the hands, feet, ankles, face, lips, mouth, or throat, difficulty breathing, sudden decrease in blood pressure, or feeling of weakness.
The following side effects may occur:
Often(may occur in 1 to 10 patients)
Reactions at the application site, rash, eruption, redness, skin inflammation (including allergic and contact dermatitis), skin swelling, itching, burning sensation.
Uncommon(may occur in 1 in 100 patients)
Generalized skin rash, allergic reactions (including hives), skin and mucous membrane swelling, anaphylactic reactions, photosensitivity.
Rare(may occur in 1 to 10 in 1000 patients)
Blistering skin, dry skin
Very rare(may occur in 1 in 10,000 patients)
Asthma attack, severe eruption, pustular rash, rash with ulcers
Frequency not known(frequency cannot be estimated from the available data) Hematoma at the application site
The absorption of diclofenac after topical application is very low, resulting in very low serum concentrations of diclofenac compared to oral administration. The likelihood of systemic side effects (such as gastrointestinal disorders, liver or kidney function disorders, bronchospasm) is therefore negligible compared to the likelihood of side effects after oral administration of diclofenac. However, if diclofenac is used for too long on large skin surfaces, systemic side effects may occur.
If any of the side effects get worse or if you notice any side effects not listed in the leaflet, you should tell your doctor or pharmacist.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309;
website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Olfen Patch
The medicine should be stored out of sight and reach of children.
You should not use Olfen Patch after the expiry date stated on the packaging.
The expiry date refers to the last day of the stated month.
After opening the packaging, the medicine can be stored for 4 months.
Do not store above 25°C.
Store in the original packaging to protect from light and drying.
Store the bag tightly closed to protect from drying.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Olfen Patch contains
The active substance of the medicine is: diclofenac sodium. Each medicinal patch contains 140 mg of diclofenac sodium.
The medicine also contains: macrogol lauryl ether, diisopropyl adipinate, glycerol, propylene glycol (E 1520), liquid sorbitol, crystallizing (E 420), sodium polyacrylate, caramelose sodium, methacrylic acid butyl copolymer, basic, colloidal silica anhydrous, light natural kaolin, sodium sulfite (E 221), disodium edetate, butylhydroxytoluene (E 321), aluminum potassium sulfate, tartaric acid, levomenthol, purified water, polyester backing layer, protective polypropylene film.
What Olfen Patch looks like and what the packaging contains
Olfen Patch is a medicinal patch with a size of 10 cm x 14 cm with a uniform layer of adhesive substance applied to the dressing, white to light brown in color; protected by a protective layer.
Olfen Patch is available in packaging containing 5 medicinal patches in 1 bag or 10 medicinal patches in 2 bags of 5 patches with the possibility of re-closing.
For more detailed information, you should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Portugal, the country of export:
Teva B.V.
Swensweg 5
2031 GA Haarlem
Netherlands
Manufacturer:
Merckle GmbH
Ludwig-Merckle-Strasse 3
89143 Blaubeuren
Germany
Teva Operations Poland Sp. z o.o
ul. Mogilska 80
31-546 Kraków
Poland
Sofarimex-Indústria Química e Farmacêutica, SA
Avenida das Indústrias, Alto de Colaride - Agualva
2735-213 Cacem
Portugal
Glaxosmithkline Santé Grand Public
23 rue Francois Jacob
92500 Rueil Malmaison
France
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Marketing authorization number in Portugal, the country of export:5128723
5128715
Parallel import authorization number: 186/23
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
France: VoltarenPlast 1%
Germany: Olfen Patch
Poland: Olfen Patch
Portugal: Olfen
Date of leaflet approval: 06.09.2023
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Teva B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Olfen PathDosage form: Gel, 10 mg/gActive substance: diclofenacManufacturer: Laboratorios Basi - Industria Farmaceutica, S.A.Prescription not requiredDosage form: Gel, 10 mg/gActive substance: diclofenacManufacturer: Sandoz GmbHPrescription not requiredDosage form: Gel, 10 mg/gActive substance: diclofenacPrescription not required
Alternatives to Olfen Path in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Olfen Path in Spain
Alternative to Olfen Path in Ukraine
Online doctors for Olfen Path
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Olfen Path – subject to medical assessment and local rules.







