
OCTOSTIM 1.5 mg/ml NASAL SPRAY SOLUTION

How to use OCTOSTIM 1.5 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Octostim 1.5 mg/ml Nasal Spray Solution
Desmopressin Acetate
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet:
- What is Octostim and what is it used for
- What you need to know before taking Octostim
- How to use Octostim
- Possible side effects
- Storage of Octostim
- Package Contents and Additional Information
1. What is Octostim and what is it used for
Octostim contains desmopressin, a natural analogue of the hypophysial hormone arginine-vasopressin, which, when administered at the appropriate dose, modifies the factors involved in blood coagulation and, as a consequence, shortens or normalizes the prolonged bleeding time (bleeding) caused by various causes.
It is indicated for:
- therapeutic treatment and prevention of bleeding (bleeding) in patients with mild Hemophilia A and von Willebrand disease who respond positively to the test dose. In exceptional cases, moderate cases of these diseases can also be treated.
- in patients who respond positively to the test dose, it decreases and normalizes the prolonged bleeding time (bleeding) due to a platelet disorder (platelet disorder).
2. What you need to know before taking Octostim
Do not use Octostim if you have wounds or irritation in the nasal mucosa, as you may not obtain the desired effect.
Do not use Octostim:
- if you are allergic to desmopressin or any of the other components of this medication (listed in section 6),
- if you have excessive prolonged thirst or thirst due to a mental disorder
- if you have heart failure and other diseases that require treatment with diuretic medications (those that increase urine production),
- in von Willebrand disease Type IIB (blood coagulation disorders), Hemophilia A, and von Willebrand disease type I with coagulant activity of factor VII below 5%, Hemophilia B, and hemophiliacs with anti-factor VIII antibodies
- if you have sodium deficiency in the blood (known hyponatremia)
- if you have inappropriate antidiuretic hormone secretion syndrome (SIADH)
- if you have uncontrolled high blood pressure.
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Octostim.
Be particularly careful with Octostim to avoid fluid accumulation in the following cases :
- children or elderly patients
- treatment with diuretic medications (those that increase urine production) (see section "Use of Other Medications").
- Coronary heart disease, hypertension
- diseases characterized by alterations in the proportion of water and electrolytes (calcium, magnesium...) in the blood
- patients at risk of increased intracranial pressure (pressure inside the skull)
- Patient with kidney problems.
Considering the possibility of excessive fluid retention, you should monitor for headache, nausea, weight gain, and blood pressure during treatment. Treatment should be discontinued in case of weight gain and signs of fluid retention/hyponatremia (decreased sodium in the blood).
Consult your doctor if you belong to any of the patient groups mentioned above.
Interaction of Octostim with Other Medications
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
This medication may interact with medications that can increase the antidiuretic effect (reduction of urine production) of desmopressin and, therefore, increase the risk of water retention and hyponatremia (decreased sodium in the blood): tricyclic antidepressants, chlorpromazine (medication for the treatment of psychiatric problems), carbamazepine (medication to prevent seizures), and chlorpropamide (medication for the treatment of diabetes mellitus) and non-steroidal anti-inflammatory drugs.
Pregnancy, Breastfeeding, and Fertility
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medication.
If you are pregnant, your doctor will assess the risk versus the benefit of treatment
Desmopressin, although in small amounts, passes into breast milk, so in the case of breastfeeding, it is recommended to replace breastfeeding.
Driving and Using Machines
The influence of Octostim on the ability to drive and use machines is nil or insignificant.
Warnings about excipients:
This medication may cause bronchospasm (sudden feeling of suffocation) because it contains benzalkonium chloride.
3. How to Use Octostim
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Octostim is administered intranasally.
Therapeutic control of bleeding or prevention of bleeding:
300 micrograms (1 spray in each nostril) are administered during bleeding (bleeding). The dose can be repeated every 12 hours for a maximum of 2-3 days. In the case of surgery, it is recommended to administer desmopressin intravenously.
It is essential that you read and follow the following instructions on how to use Octostim
Before using Octostim for the first time, you must prepare the pump by pressing it 4 times, or until a spray is obtained. If you have not used Octostim during the past week, it is necessary to prepare the pump again by pressing it once, or until a spray appears.
IMPORTANT! When using Octostim, the end of the tube must always be submerged in the liquid.
INSTRUCTIONS FOR USE:
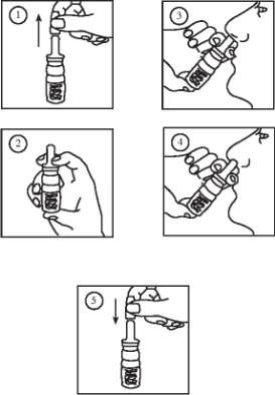
- Remove the protective cap from the applicator.
- Hold the bottle with your thumb, index, and middle fingers as indicated in figure 2.
- Slightly tilt your head back while inserting the applicator directly into the nostril as indicated in figure 3. Start nebulization by covering the other nostril simultaneously. Inhale the product gently in each dose.
- Repeat the administration in the other nostril
- Replace the protective cap. Store the bottle always in a vertical position.
If you use more Octostim than you should
If you have used more Octostim than you should, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 915 620 420, indicating the medication and the amount used.
If you use Octostim more than you should or accidentally, the symptoms would be those consequent to water retention and/or decreased sodium in the blood, such as headache, nausea, vomiting, weight gain, and in severe cases, convulsions.
Treatment should be discontinued, and fluid intake restricted, and symptomatic treatment if necessary.
If you forget to take Octostim
If you miss a dose, apply the next dose at the usual time. Do not take a double dose to make up for the missed doses.
If you interrupt treatment with Octostim
Your doctor will indicate the duration of your treatment with Octostim. Do not stop treatment before, as it may not have the expected effect.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, Octostim can cause side effects, although not everyone will experience them.
The following side effects are frequent, may affect up to 1 in 10 people:
- headache
- stomach pain
- nausea
- nasal congestion/rhinitis
- nasal bleeding
- transient tachycardia
- facial flushing
- eye redness
The following side effects are very rare, may affect up to 1 in 10,000 people:
- sodium deficiency in the blood (hyponatremia)
Other side effects for which the frequency is not known:
- weight gain, vomiting, muscle spasms, swelling, fatigue, confusion, dizziness, and in severe cases, convulsions and allergic reactions to benzalkonium chloride (preservative)
- redness and itching.
.
If you consider that any of the side effects you are experiencing is serious or if you notice any side effect not mentioned in this package leaflet, inform your doctor or pharmacist.
Reporting of Side Effects:
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: http://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Octostim
Keep this medication out of the sight and reach of children.
Do not use Octostim after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
The validity period after the first opening of the packaging is 6 months.
Do not store above 25°C.
Always store the bottle in a vertical position.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Octostim:
The active ingredient is desmopressin acetate. 1 milliliter of nasal spray solution contains 1.5 milligrams of desmopressin acetate, equivalent to 1.34 milligrams of desmopressin.
The other components are:
Benzalkonium chloride
Citric acid monohydrate,
Sodium chloride,
Disodium phosphate dihydrate,
Purified water.
Appearance of the Product and Package Contents
Octostim is presented in 10-milliliter brown glass bottles containing 2.5 milliliters of solution, which are equipped with pre-compressed aerosol pumps with an applicator and a protective cap, designed to release 150 micrograms each time the pump is pressed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
FERRING S.A.U.
C/ Arquitecto Sánchez Arcas 3, 1º.
28040 Madrid
SPAIN
Manufacturer
FERRING GmbH
Wittland, 11
24109 Kiel
GERMANY
Date of the Last Revision of this Package Leaflet:July 2022
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OCTOSTIM 1.5 mg/ml NASAL SPRAY SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 360mcg/ml desmopressin (anhydrous base)Active substance: desmopressinManufacturer: Laboratorio Reig Jofre, S.A.Prescription requiredDosage form: SUBLINGUAL TABLET, 120 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: SUBLINGUAL TABLET, 240 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription required
Online doctors for OCTOSTIM 1.5 mg/ml NASAL SPRAY SOLUTION
Discuss questions about OCTOSTIM 1.5 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















