NEVANAC 1 mg/ml EYE DROPS, SUSPENSION

How to use NEVANAC 1 mg/ml EYE DROPS, SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
NEVANAC 1 mg/ml eye drops, suspension
nepafenac
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is NEVANAC and what is it used for
- What you need to know before you use NEVANAC
- How to use NEVANAC
- Possible side effects
- Storing NEVANAC
- Contents of the pack and other information
1. What is NEVANAC and what is it used for
NEVANAC contains the active substance nepafenac which belongs to a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs).
NEVANAC is used in adults:
To prevent and relieve eye pain and inflammation after cataract surgery in the eye.
To reduce the risk of macular edema (swelling in the back of the eye) after cataract surgery in the eye in diabetic patients.
2. What you need to know before you use NEVANAC
- if you are allergic to nepafenac or any of the other ingredients of this medicine (listed in section 6),
- if you are allergic to other non-steroidal anti-inflammatory drugs (NSAIDs)
- if you have had asthma, skin allergy, or severe nasal inflammation when using other NSAIDs. Examples of NSAIDs are: acetylsalicylic acid, ibuprofen, ketoprofen, piroxicam, and diclofenac.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use NEVANAC.
- if you bruise easily or have bleeding problems or have had them in the past.
- if you have any other eye disorder (e.g. an eye infection) or if you are using other eye medicines (especially eye steroids).
- if you have diabetes.
- if you have rheumatoid arthritis.
- if you have had several eye operations in a short time.
Avoid exposure to sunlight during treatment with NEVANAC.
It is not recommended to wear contact lenses after cataract surgery. Your doctor will tell you when you can start wearing contact lenses again (see also “NEVANAC contains benzalkonium chloride”).
Children and adolescents
Do not use this medicine in children and adolescents under 18 years old as the safety and efficacy in this population have not been established.
Other medicines and NEVANAC
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
NEVANAC may affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma.
Tell your doctor if you are using medicines that reduce blood clotting (warfarin) or other NSAIDs. These medicines may increase the risk of bleeding.
Pregnancy and breastfeeding
If you are pregnant or might become pregnant, consult your doctor before using NEVANAC. Women who may become pregnant are advised to use effective contraceptive methods during treatment with NEVANAC. The use of NEVANAC is not recommended during pregnancy. Do not use NEVANAC unless clearly indicated by your doctor.
If you are breastfeeding, NEVANAC may pass into breast milk. However, no effects on the breastfed child are expected. NEVANAC can be used during breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Do not drive or use machines until your vision is clear. Immediately after applying NEVANAC, you may notice that your vision becomes blurred.
NEVANAC contains benzalkonium chloride
This medicine contains 0.25 mg of benzalkonium chloride in each 5 ml equivalent to 0.05 mg/ml.
NEVANAC contains a preservative, benzalkonium chloride, which may be absorbed by soft contact lenses and may alter the color of contact lenses. Remove contact lenses before using this medicine and wait 15 minutes before putting them back. Benzalkonium chloride may cause eye irritation, especially if you have dry eyes or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medicine.
3. How to use NEVANAC
Follow exactly the instructions of administration of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Use NEVANAC only in your eyes. Do not swallow or inject.
The recommended dose is
One drop in the affected eye(s), three times a day, morning, noon, and night. Use it at the same time every day.
When to use and for how long
Start 1 day before cataract surgery. Continue on the day of surgery. Then use it for the period of time that your doctor indicates, which may be up to 3 weeks (to prevent and relieve eye pain and inflammation) or 60 days (to prevent macular edema) after your surgery.
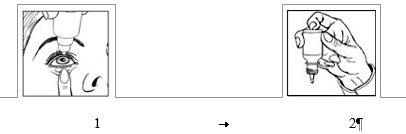
How to use
Wash your hands before starting.
|
- Shake well before use.
- Remove the cap from the bottle.
- After removing the cap, the security seal ring must be removed before using this medicine.
- Hold the bottle, upside down, between your fingers.
- Tilt your head back.
- Gently pull down the lower eyelid to form a pouch (Figure 1).
- Bring the tip of the bottle close to the eye. You can use a mirror to help.
- Do not touch the eye, eyelid, or surrounding areas with the dropper because the drops could become contaminated.
- Gently press the base of the bottle to release one drop of NEVANAC at a time.
- Do not squeeze the bottle: it is designed to deliver one drop with a gentle pressure on the base (Figure 2).
If you are applying drops to both eyes, repeat the above steps for the other eye. Replace the cap on the bottle immediately after use.
If a drop falls outside the eye, try again.
If you are using other eye drops, wait at least 5 minutes between applying NEVANAC and the other drops.
If you use more NEVANAC than you should
Contact your doctor for detailed instructions. Do not apply more drops until it is time for your next dose.
If you forget to use NEVANAC
Apply a single dose as soon as you remember. If it is almost time for your next dose, do not apply the missed dose. Continue with your regular dosing schedule. Do not use a double dose to make up for missed doses. Do not apply more than one drop 3 times a day in the affected eye(s).
If you stop using NEVANAC
Do not stop using NEVANAC without consulting your doctor. You can usually continue using the eye drops unless the side effects are severe. If these effects worry you, talk to your doctor or pharmacist.
If you have any other questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You may have a higher risk of experiencing corneal side effects (problems on the surface of the eye), if you:
- have had a complicated eye operation
- have had several eye operations in a short time
- have certain conditions affecting the surface of the eye, such as inflammation or dry eyes
- have certain diseases such as diabetes or rheumatoid arthritis
Contact your doctor immediately if your eyes become red or if the pain increases while using the drops. This may be due to inflammation of the surface of the eye with or without loss or damage to cells or inflammation of the colored part of the eye (iritis). These side effects have been observed in up to 1 in 100 people.
The following side effects have also been observed with NEVANAC 1 mg/ml eye drops, suspension or NEVANAC 3 mg/ml eye drops, suspension or with both:
Uncommon(may affect up to 1 in 100 people)
- Eye effects:inflammation of the surface of the eye with or without cell damage or loss, feeling of something in the eye, crusts or drooping of the eyelid.
Rare(may affect up to 1 in 1,000 people)
- Eye effects:inflammation of the iris, eye pain, eye discomfort, dry eyes, eyelid swelling, eye irritation, itching in the eyes, eye discharge, allergic conjunctivitis (eye allergy), increased tearing, deposits on the surface of the eye, fluid accumulation or swelling in the back of the eye, eye redness.
- General side effects:dizziness, headache, symptoms of allergy (allergic swelling of the eyelids), nausea, itching, redness, and inflammation of the skin.
Frequency not known(frequency cannot be estimated from the available data)
- Eye effects:damage to the surface of the eye such as thinning or perforation, worsening of eye healing, scarring on the surface of the eye, blurred vision, reduced vision, eye swelling, blurred vision.
- General side effects:vomiting, increased blood pressure
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing NEVANAC
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and carton after EXP. The expiry date is the last day of the month shown.
Do not store above 30°C.
To avoid infections, discard the bottle 4 weeks after first opening. Write the date of opening on the space provided on the bottle label and carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
- The active substance is nepafenac. One ml of suspension contains 1 mg of nepafenac.
- The other ingredients are benzalkonium chloride (see section 2), carbomer, disodium edetate, mannitol, purified water, sodium chloride, and tyloxapol.
Small amounts of sodium hydroxide and/or hydrochloric acid are added to maintain normal acidity levels (pH levels).
Appearance and pack contents
NEVANAC is a liquid (a pale yellow to orange suspension) supplied in a carton containing a 5 ml plastic bottle with a screw cap.
Marketing authorisation holder
Novartis Europharm Limited
Vista Building
Elm Park,
Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Manufacturing NV
Rijksweg 14
2870 Puurs-Sint-Amands
Belgium
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can request more information about this medicine from the local representative of the marketing authorisation holder.
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceska republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
| Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: + 421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
| Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA “Novartis Baltics” Tel: +371 67 887 070 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NEVANAC 1 mg/ml EYE DROPS, SUSPENSIONDosage form: EYE DROP, 3 mg/mlActive substance: nepafenacManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 3 mg/mlActive substance: nepafenacManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: ketorolacManufacturer: Abbvie Spain, S.L.U.Prescription required
Online doctors for NEVANAC 1 mg/ml EYE DROPS, SUSPENSION
Discuss questions about NEVANAC 1 mg/ml EYE DROPS, SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions