LIVMARLI 9.5 mg/mL ORAL SOLUTION

How to use LIVMARLI 9.5 mg/mL ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Livmarli 9.5 mg/ml Oral Solution
maralixibat
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you or your child experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Livmarli and what is it used for
- What you need to know before you or your child start taking Livmarli
- How to take Livmarli
- Possible side effects
- Storage of Livmarli
- Contents of the pack and other information
1. What is Livmarli and what is it used for
What is Livmarli
Livmarli contains the active substance maralixibat (as chloride). It helps to remove substances called bile acids from the body.
Bile acids are found in the digestive fluid called bile that the liver produces. Bile acids pass from the liver to the intestine and help digest food. After helping with digestion, they return to the liver.
What Livmarli is used for
Livmarli is used to treat cholestatic pruritus in patients from 2 months of age with Alagille syndrome (ALGS). Livmarli is also used to treat progressive familial intrahepatic cholestasis (PFIC) in patients from 3 months of age.
ALGS and PFIC are rare genetic diseases that can cause bile acids to build up in the liver. This is called cholestasis. Cholestasis can get worse over time and often causes intense itching, fatty deposits under the skin (xanthomas), growth delay, and feeling tired.
How Livmarli works (maralixibat)
Maralixibat works by reducing the buildup of bile acids in the liver. It does this by preventing bile acids from being transported back to the liver once they have done their job in the intestines. This allows bile acids to leave the body through the feces.
2. What you need to know before you or your child start taking Livmarli
Do not use Livmarli
- If you or your child are allergic to maralixibat or any of the other ingredients of this medicine (listed in section 6).
- If you or your child have severe kidney and/or liver problems.
Warnings and precautions
Tell your doctor if your diarrhea gets worse while taking Livmarli. If you have diarrhea, drink plenty of fluids to avoid dehydration.
An increase in liver enzyme levels may be seen in liver function tests while taking Livmarli. Before starting Livmarli, your doctor will measure your liver function to check if your liver is working properly. Your doctor will perform regular checks to monitor liver function.
Your doctor may do blood tests before starting or during treatment with Livmarli to check your INR (International Normalized Ratio; a laboratory test to monitor the risk of bleeding) and levels of certain fat-soluble vitamins (vitamin A, D, E, and K). If your vitamin levels are low, your doctor may recommend that you take vitamins.
Certain diseases, medicines, or operations can affect the movement of food through the intestines. They can also affect the movement of bile acids between the liver and the intestines. This can affect how maralixibat works.
Make sure your doctor knows about any diseases, medicines, or operations you have had or taken.
Taking Livmarli with medicines that contain alcohol may cause side effects in children under 5 years of age or in patients with reduced liver and/or kidney function. If you or your child have reduced liver and/or kidney function or if your child is under 5 years of age, talk to your doctor or pharmacist before starting to use this medicine, especially if you or your child use other medicines or dietary supplements that contain propylene glycol or alcohol.
Children
Livmarli is not recommended in children with Alagille syndrome under 2 months of age. The reason is that it is not yet known if it is safe and effective in this age group.
Other medicines and Livmarli
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those without a prescription and herbal medicines.
Tell your doctor if you are taking any of the following medicines:
- Fluvastatin, rosuvastatin, or simvastatin (medicines used to treat high cholesterol levels in the blood)
- Midazolam (a medicine used for sedation or to induce sleep)
- Ursodeoxycholic acid (a medicine used to treat liver diseases)
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. If you are pregnant, it is best not to take Livmarli.
Livmarli does not enter the bloodstream, so it is not expected to pass into breast milk. However, always follow the advice of your doctor.
Driving and using machines
Livmarli has no or negligible influence on the ability to drive and use machines.
Livmarli contains propylene glycol and sodium
This medicine contains 364.5 mg of propylene glycol in each ml. When taken as recommended for ALGS, the exposure to propylene glycol will be up to 17 mg/kg/day. When taken as recommended for PFIC, the exposure to propylene glycol will be up to 50 mg/kg/day.
If your child is under 5 years of age, talk to your doctor or pharmacist before starting to give them this medicine, especially if they use other medicines that contain propylene glycol or alcohol. If you are pregnant or breastfeeding, or if you have liver or kidney disease, do not take this medicine unless your doctor recommends it. Your doctor may do additional checks while you are taking this medicine.
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially “sodium-free”.
3. How to take Livmarli
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again.
How much to take
- The dose of Livmarli that you will be given depends on your body weight. Your doctor will calculate the dose and tell you how much to take and what size of oral syringe to use. Your doctor will also write this information and other relevant details (e.g., your weight) in a special patient booklet. Bring the patient booklet with you every time you visit your doctor. Do not calculate the dose yourself and only take the dose that your doctor calculates for you. The doses of maralixibat given to patients with ALGS and with PFIC are different. Your doctor will make sure to select the correct dose for you, depending on your disease and body weight.
- For ALGS: the target dose is 380 micrograms of maralixibat per kilogram of body weight once a day.
-The initial dose is 190 micrograms per kilogram of body weight once a day.
-The dose will be increased to 380 micrograms per kilogram of body weight once a day after one week. Your doctor will tell you when you can increase the dose. Your doctor will also tell you how much to take and what size of oral syringe to use for a higher dose.
- For PFIC: the initial dose is 285 micrograms per kilogram of body weight once a day, in the morning.
- This dose may be increased to 285 micrograms per kilogram of body weight twice a day and then to 570 micrograms per kilogram of body weight twice a day, depending on how well it is tolerated.
- Patients under 5 years of age and patients with moderate reduction of liver or kidney function should not take doses higher than 285 micrograms per kilogram of body weight twice a day. Your doctor will tell you if this dose restriction applies to you or your child.
Taking the medicine
You can take Livmarli with or without food, up to 30 minutes before eating, in the morning.
Give the dose in the mouth using the oral syringe and swallow it (see Figure M). Do not mix the oral solution with food or drinks.
Use the following table to make sure you use the correct size of oral syringe for the prescribed dose:
Dose volume prescribed (ml) | Oral syringe size (ml) |
0.1 to 0.5 | 0.5 |
0.6 to 1 | 1 |
1.25 to 3 | 3 |
Make sure to measure the volume carefully to avoid an overdose.
How to take a dose of this medicine
Step 1: Withdrawal of the dose
1.1To open the bottle, remove the child-resistant cap by pressing it firmly downwards and turning it to the left (counterclockwise) (see Figure A). Do not throw away the child-resistant cap because you will need to put it back after withdrawing the dose you need.
Figure A
1.2Make sure you use the correct size of oral syringe for the prescribed dose (see table above). Your doctor will tell you what size of syringe to use.
- If you use a new oral syringe, take it out of the wrapper (see Figure B). Throw the wrapper away in the household trash.
- If you use an oral syringe that has been used before, make sure it is clean and dry (see 2.4 cleaning instructions).
Figure B
- If the oral syringe has a cap, remove it and throw it away in the household trash (see Figure C).
Figure C
The syringe has dose marks on the cylinder. One end of the syringe has a cone that fits into the medicine bottle. The other end of the syringe has a wing and a plunger that is used to withdraw the medicine from the syringe and give it (see Figure D).
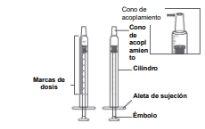
Figure D
1.3Push the plunger all the way down to remove air from the syringe (see Figure E).

Figure E
1.4Make sure to remove the child-resistant cap from the bottle and insert the cone of the syringe into the bottle in an upright position. The cone of the syringe should fit snugly into the bottle opening

Figure F
1.5Once the syringe is in place, turn the bottle upside down (see Figure G).
Figure G
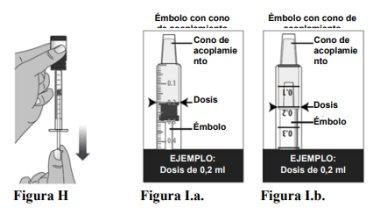
1.6To withdraw a dose from the bottle, slowly pull the plunger back until the plunger is aligned with the mark on the syringe cylinder that matches the prescribed dose (see Figure H). There are two types of plungers that you may receive with the syringe: a plunger with a flat cone or a plunger with a pointed cone (see Figure I below step 1.6). See Figure I to know how to align the plunger with the prescribed dose. For the plunger with the flat cone, the flat end of the plunger should be aligned with the mark on the cylinder that matches the prescribed dose (Figure I.a.). For the plunger with the pointed cone, make sure the flat and wide part located below the cone is aligned with the correct mark (Figure I.b.).
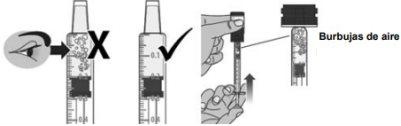
1.7Check the syringe for air bubbles. If you see air bubbles:
- Press the plunger to push the air bubbles back into the bottle (see Figure J).
- Then, withdraw the prescribed dose again following the instructions in step 1.6.
| |
Figure J.a. Check for air bubbles | Figure J.b. Press the plunger on the syringe to remove air bubbles. |
1.8Once you have withdrawn the correct dose without air bubbles, leave the syringe in the bottle and turn the bottle right side up (see Figure K).
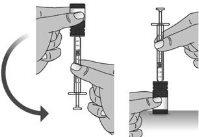
Figure K
1.9Carefully remove the syringe from the bottle (see Figure L). To do this, hold the bottle firmly with one hand and the syringe by the cylinder with the other.
- Do not press the plunger on the syringe during this step.
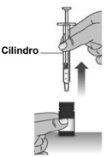
Figure L
Step 2: Administration of the dose
Note:You or your child should be standing while taking the dose and for a few minutes after.
2.1Place the cone of the oral syringe against the inside of the cheek (see Figure M).
Slowly press the plunger all the way down and gently pour the oral solution into the mouth (see Figure N).
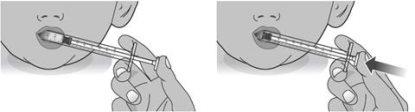
Figure M Figure N
2.2Make sure you or the child swallows the dose. If you are not sure if the whole dose has been swallowed, do not give another dose. Wait until it is time for the next dose.
2.3To close the bottle,screw the child-resistant cap back onto the bottle by turning it to the right (clockwise) (see Figure O).
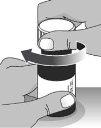
Figure O
2.4Remove the plunger from the syringe cylinder (see Figure P) and wash it with water after each use. Let the plunger air dry before using it again.
Figure P
- Oral syringes can be rinsed with water, left to air dry, and reused for 130 days.
If you take more Livmarli than you should
Tell your doctor if you take more Livmarli than you should.
If you forget to take Livmarli
If you have forgotten to take a dose, take the next dose at the usual time.
If you stop taking Livmarli
Do not stop taking Livmarli without talking to your doctor first.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may happen with this medicine.
Very common(may affect more than 1 in 10 people)
- Diarrhea
- Stomach pain (abdominal) (ALGS)
Common(may affect up to 1 in 10 people)
- Stomach pain (abdominal) (PFIC)
- Increased liver enzymes (ALT, AST)
These side effects are usually mild or moderate and may improve during continued treatment with Livmarli.
If you experience any other side effects, tell your doctor.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Livmarli
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage temperature. Store in the original packaging to protect it from light.
Do not use this medicine after the expiry date stated on the carton and on the bottle after "EXP". The expiry date is the last day of the month indicated.
Once the bottle is opened, it should be stored below 30 °C and the medicine should be administered within 130 days of opening. After these 130 days, the bottle should be discarded, even if it is not empty. Note the opening date on the Livmarli bottle.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Livmarli Composition
- The active substance is maralixibat (in the form of chloride).
- Each ml of solution contains maralixibat chloride equivalent to 9.5 mg of maralixibat.
- The other ingredients are propylene glycol (E1520) (see section 2 "Livmarli contains propylene glycol and sodium"), disodium edetate (see section 2 "Livmarli contains propylene glycol and sodium"), sucralose, grape flavor, and purified water.
Product Appearance and Package Contents
Livmarli is a clear and colorless to light yellow oral solution. It is stored in 30 ml amber plastic bottles with a pre-installed adapter and a child-resistant closure with a foam seal. The 3 sizes of oral syringes (0.5 ml, 1 ml, and 3 ml) supplied in the package are compatible with the pre-installed adapter and the resealable bottle cap. To ensure the correct dose of Livmarli is administered, refer to the table in section 3 ("How to take Livmarli") to select the correct oral syringe size.
Package Size
1 bottle of 30 ml with 3 oral syringes (0.5 ml, 1 ml, and 3 ml).
Marketing Authorization Holder
Mirum Pharmaceuticals International B.V.
Kingsfordweg 151
1043 GR Amsterdam,
Netherlands
Manufacturer
Millmount Healthcare Limited
Block 7 City North Business Campus
Stamullen, Co. Meath, K32 YD60
Ireland
Date of Last Revision of this Leaflet:
This medicine has been authorized under "exceptional circumstances". This means that due to the rarity of this disease, it has not been possible to obtain complete information on this medicine.
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LIVMARLI 9.5 mg/mL ORAL SOLUTIONDosage form: CAPSULE, 1200 µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription requiredDosage form: CAPSULE, 200 µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription requiredDosage form: CAPSULE, 200 - REVIEW µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription required
Online doctors for LIVMARLI 9.5 mg/mL ORAL SOLUTION
Discuss questions about LIVMARLI 9.5 mg/mL ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions