BYLVAY 400 micrograms hard capsules

How to use BYLVAY 400 micrograms hard capsules
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information: Summary of Product Characteristics
Bylvay 200 micrograms hard capsules
Bylvay 400 micrograms hard capsules
Bylvay 600 micrograms hard capsules
Bylvay 1200 micrograms hard capsules
odevixibat
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet (see section 4).
Contents of the pack
- What is Bylvay and what is it used for
- What you need to know before you take Bylvay
- How to take Bylvay
- Possible side effects
- Storage of Bylvay
- Contents of the pack and other information
1. What is Bylvay and what is it used for
Bylvay contains the active substance odevixibat. Odevixibat is a medicine that increases the elimination of certain substances called bile acids from the body. Bile acids are components of the digestive fluid called bile, which is produced by the liver and secreted into the intestine. Odevixibat blocks the mechanism that normally reabsorbs them from the intestine after they have done their job. This allows them to be eliminated from the body through the feces.
Bylvay is used to treat progressive familial intrahepatic cholestasis (PFIC) in patients aged 6 months or older. PFIC is a liver disease caused by the accumulation of bile acids (cholestasis) that worsens over time and is often accompanied by severe itching.
2. What you need to know before you take Bylvay
Do not take Bylvay
- if you are allergic to odevixibat or any of the other ingredients of this medicine (listed in section 6)
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Bylvay:
- if you have been diagnosed with a complete absence or lack of function of the bile salt export pump protein;
- if you have severe liver dysfunction;
- if you have reduced stomach or intestinal motility or reduced bile acid circulation between the liver, bile, and small intestine due to medications, surgical interventions, or diseases other than PFIC, as this may reduce the effect of odevixibat.
Consult your doctor if you experience diarrhea while taking Bylvay. It is recommended to drink sufficient liquid to patients with diarrhea to avoid dehydration.
During treatment with Bylvay, elevated liver enzyme levels may be observed in liver function tests. Before starting treatment with Bylvay, your doctor will measure your liver function to check the functioning of your liver. Your doctor will perform regular checks to monitor your liver function.
Before and during treatment, your doctor may also check your blood levels of vitamins A, D, and E and your INR (international normalized ratio, which measures your risk of bleeding).
Children
The use of Bylvay is not recommended in infants under 6 months of age, as it is not known whether the medicine is safe and effective in this age group.
Using Bylvay with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Treatment with odevixibat may affect the absorption of fat-soluble vitamins, such as vitamins A, D, and E, and some medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, consult your doctor before using this medicine.
The use of Bylvay is not recommended during pregnancy or in women of childbearing potential who are not using contraceptive methods.
It is not known whether odevixibat can pass into breast milk and affect the baby. Your doctor will help you decide whether to stop breastfeeding or avoid treatment with Bylvay, taking into account the benefit of breastfeeding for the baby and the benefit of Bylvay for the mother.
Driving and using machines
Bylvay has no or negligible influence on the ability to drive and use machines.
3. How to take Bylvay
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Treatment should be initiated and supervised by a doctor with experience in the treatment of progressive liver disease with reduced bile flow.
The dose of Bylvay depends on your weight. Your doctor will calculate the correct number of capsules you should take and their concentration.
The recommended dose is
- 40 micrograms of odevixibat per kilogram of body weight once daily
- If the medicine does not work well enough after 3 months, your doctor may increase the dose to 120 micrograms of odevixibat per kilogram of body weight (up to a maximum of 7,200 micrograms once daily).
Different doses are not recommended for adults.
Method of administration
Take the capsules once daily in the morning, with or without food.
All capsules can be swallowed whole with a glass of water or opened and sprinkled on some food or in a liquid suitable for the age (e.g., breast milk, formula, or water).
The larger capsules, 200 and 600 micrograms, are designed to be opened and sprinkled on some food or in a liquid suitable for the age, but can be swallowed whole. The smaller capsules, 400 and 1,200 micrograms, are designed to be swallowed whole but can be opened and sprinkled on some food or in a liquid suitable for the age.
You will find detailed instructions on how to open the capsules and sprinkle the contents on some food or in a liquid at the end of this leaflet.
If the medicine does not improve your disease after 6 months of continuous daily treatment, your doctor will recommend an alternative treatment.
If you take more Bylvay than you should
Tell your doctor if you think you have taken too much Bylvay.
Possible symptoms of an overdose are diarrhea and stomach and intestine problems.
If you forget to take Bylvay
Do not take a double dose to make up for a forgotten dose. Take the next dose at the usual time.
If you stop taking Bylvay
Do not stop taking Bylvay without talking to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects may occur with the following frequencies:
Very common(may affect more than 1 in 10 people)
- diarrhea, including bloody diarrhea, soft stools
- vomiting
- abdominal pain (stomach pain)
Common(may affect up to 1 in 10 people)
- increased liver size
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet (see section 4). You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Bylvay
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date is the last day of the month shown.
Store in the original package to protect from light. Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Bylvay Composition
- The active ingredient is odevixibat.
Each hard capsule of Bylvay 200 micrograms contains 200 micrograms of odevixibat (as sesquihydrate).
Each hard capsule of Bylvay 400 micrograms contains 400 micrograms of odevixibat (as sesquihydrate).
Each hard capsule of Bylvay 600 micrograms contains 600 micrograms of odevixibat (as sesquihydrate).
Each hard capsule of Bylvay 1,200 micrograms contains 1,200 micrograms of odevixibat (as sesquihydrate).
The other ingredients are:
- Capsule content
Microcrystalline cellulose
Hypromellose
Capsule shell
Bylvay 200 micrograms and 600 micrograms hard capsules
Hypromellose
Titanium dioxide (E171)
Yellow iron oxide (E172)
Bylvay 400 micrograms and 1,200 micrograms hard capsules
Hypromellose
Titanium dioxide (E171)
Yellow iron oxide (E172)
Red iron oxide (E172)
Printing ink
Lacquer
Propylene glycol
Black iron oxide (E172)
Product Appearance and Container Contents
Bylvay 200 micrograms hard capsules:
Capsules of size 0 (21.7 mm × 7.64 mm) with an opaque ivory-colored cap and an opaque white-colored body; with the inscription «A200» in black ink.
Bylvay 400 micrograms hard capsules:
Capsules of size 3 (15.9 mm × 5.82 mm) with an opaque orange-colored cap and an opaque white-colored body; with the inscription «A400» in black ink.
Bylvay 600 micrograms hard capsules:
Capsules of size 0 (21.7 mm × 7.64 mm) with an opaque ivory-colored cap and body; with the inscription «A600» in black ink.
Bylvay 1,200 micrograms hard capsules:
Capsules of size 3 (15.9 mm × 5.82 mm) with an opaque orange-colored cap and body; with the inscription «A1200» in black ink.
The hard capsules of Bylvay are packaged in a plastic bottle with a polypropylene closure, with a security seal and child-resistant closure. Package size: 30 hard capsules.
Marketing Authorization Holder
Ipsen Pharma
70 rue Balard
75015 Paris
France
Manufacturer
Almac Pharma Services Limited
Seagoe Industrial Estate
Portadown, Craigavon
County Armagh
BT63 5UA
United Kingdom (Northern Ireland)
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien/Luxembourg/ Luxemburg Ipsen NV België/Belgique/Belgien Tél/Tel: +32 9 243 96 00 | Italia Ipsen SpA Tel: + 39 02 39 22 41 |
| Latvija Ipsen Pharma representative office Tel: + 371 67622233 |
| Lietuva Ipsen Pharma SAS Lietuvos filialas Tel: +370 700 33305 |
Danmark, Norge, Suomi/Finland, Sverige, Ísland Institut Produits Synthèse (IPSEN) AB Sverige/Ruotsi/Svíþjóð Tlf/Puh/Tel/Sími: +46 8 451 60 00 | Magyarország IPSEN Pharma Hungary Kft. Tel.: + 36 1 555 5930 |
Deutschland, Österreich Ipsen Pharma GmbH Deutschland Tel: +49 89 2620 432 89 | Nederland Ipsen Farmaceutica B.V. Tel: +31 (0) 23 554 1600 |
Eesti Centralpharma Communications OÜ Tel: +372 60 15 540 | Polska Ipsen Poland Sp. z o.o. Tel.: + 48 22 653 68 00 |
Ελλάδα, Κύπρος, Malta Ipsen Μονοπρόσωπη EΠΕ Ελλάδα Τηλ: +30 210 984 3324 | Portugal Ipsen Portugal - Produtos Farmacêuticos S.A. Tel: + 351 21 412 3550 |
España Ipsen Pharma, S.A.U. Tel: +34 936 858 100 | România Ipsen Pharma România SRL Tel: + 40 21 231 27 20 |
France Ipsen Pharma Tél: +33 1 58 33 50 00 | Slovenija Swixx Biopharma d.o.o. Tel: + 386 1 2355 100 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | Slovenská republika Ipsen Pharma, organizačná zložka Tel: + 420 242 481 821 |
Ireland, United Kingdom (Northern Ireland) Ipsen Pharmaceuticals Limited Tel: +44 (0)1753 62 77 77 |
Date of Last Revision of this Leaflet:
This medicinal product has been authorized under exceptional circumstances. This means that due to the rarity of this disease, it has not been possible to obtain complete information on this medicinal product.
The European Medicines Agency will review any new information that may become available every year and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
There are also links to other websites on rare diseases and orphan medicines.
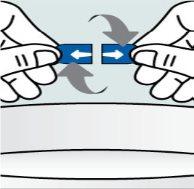
Instructions
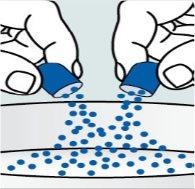
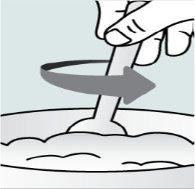
Instructions for opening the capsules and sprinkling the contents over some food:
Step 1. Place a small amount of soft food in a bowl (2 tablespoons/30 ml of yogurt, apple sauce, mashed banana or carrot, chocolate pudding, rice pudding, or oatmeal). The food should be at room temperature or cooler.
| Step 2:
|
| Step 3:
|
| Step 4:
|
|
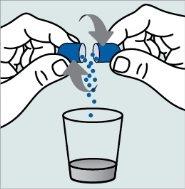
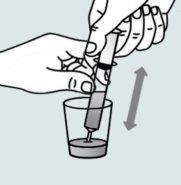
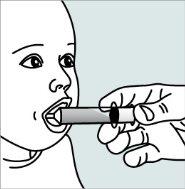
Instructions for opening the capsules and sprinkling the contents into a suitable liquid for the age:
Do not administer the medicinal product through a baby bottle or a medicine cup, as the granules will not pass through the opening. The granules will not dissolve in liquids.
Contact your pharmacy if you do not have a suitable oral syringe to administer the medicinal product at home.
| Step 1:
|
| |
| Step 2:
|
Step 3:
| |
| Step 4:
|
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BYLVAY 400 micrograms hard capsulesDosage form: CAPSULE, 1200 µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription requiredDosage form: CAPSULE, 200 µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription requiredDosage form: CAPSULE, 600 µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription required
Online doctors for BYLVAY 400 micrograms hard capsules
Discuss questions about BYLVAY 400 micrograms hard capsules, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions