LITAK 2 mg/ml INJECTABLE SOLUTION

How to use LITAK 2 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
LITAK 2 mg/ml solution for injection
cladribine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What LITAK is and what it is used for
- What you need to know before you use LITAK
- How to use LITAK
- Possible side effects
- Storing LITAK
- Contents of the pack and other information
1. What is LITAK and what is it used for
LITAK contains the active substance cladribine. Cladribine is a cytostatic that affects the growth of malignant white blood cells (cancerous), which play an important role in hairy cell leukemia (tricholeukemia). LITAK is used to treat this disease.
2. What you need to know before you use LITAK
Do not use LITAK
- If you are allergic to cladribine or any of the other ingredients of LITAK (listed in section 6).
- If you are pregnant or breastfeeding.
- If you are under 18 years old.
- If you have moderate to severe kidney or liver failure.
- If you are using other medicines that affect blood cell production in the bone marrow (myelosuppression).
Warnings and precautions
Talk to your doctor or pharmacist before starting treatment with LITAK.
At any time during or after treatment, tell your doctor or nurse immediatelyif you experience blurred vision, loss of vision, or double vision, difficulty speaking, weakness in one arm or leg, a change in your walking or balance problems, persistent numbness, decreased sensitivity, or loss of sensitivity, memory loss, or confusion. These can be symptoms of a serious and potentially life-threatening brain diseaseknown as multifocal leukoencephalopathy (LMP).
If you had any of these symptoms before starting treatment with cladribine, tell your doctorif you notice any change in these symptoms.
Tell your doctor if you have or have had:
- Kidney or liver problems.
- Infections
- If you get an infection, it should be treated before starting LITAK.
- If you notice any signs of infection (e.g., flu-like symptoms or fever) during or after treatment with LITAK, tell your doctor immediately.
- Fever.
Before starting treatment with LITAK, and during treatment, you will have regular blood tests to check if you can continue treatment safely. Your doctor may decide that you receive blood transfusions to improve your blood cell count. Additionally, your liver and kidney function will be monitored.
Men who wish to have children should inform their doctor before starting treatment with LITAK. You should not father a child during treatment and up to six months after completing treatment with LITAK. Your doctor may advise you on the possibility of preserving sperm by freezing (cryopreservation).
Other medicines and LITAK
Tell your doctor if you are taking or have recently taken any other medicines, including those obtained without a prescription. In particular, tell your doctor if you are taking any medicines that contain:
- Corticosteroids, often used to treat inflammation.
- Antivirals, used to treat viral infections.
DO NOT use LITAK with other medicines that affect blood cell production in the bone marrow (myelosuppression).
Pregnancy and breastfeeding
Do not use LITAK if you are pregnant. You should use adequate contraceptive precautions during treatment and at least six months after the last dose of LITAK. If you become pregnant during treatment, tell your doctor immediately.
Do not breastfeed while being treated with LITAK and for at least six months after receiving the last dose of LITAK.
Driving and using machines
LITAK's influence on the ability to drive and use machines is significant. If you feel drowsy, which may occur due to the low red blood cell count caused by treatment with LITAK, or dizzy, do not drive or use machines.
3. How to use LITAK
Always follow the instructions for administering LITAK as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Your doctor will calculate the dose you should receive based on your body weight and will explain the treatment schedule in detail. The recommended daily dose is 0.14 mg per kg of body weight, for five consecutive days (single treatment cycle).
LITAK must be injected under the skin (subcutaneous injection), approximately at the same time each day. If you inject LITAK yourself, you must first receive proper training from your doctor or nurse. You will find detailed instructions for injection at the end of this leaflet.
You may also receive an additional medicine containing the active substance alopurinol to reduce excess uric acid.
If you use more LITAK than you should
In case of injecting an incorrect dose, tell your doctor immediately.
If you forget to use LITAK
Do not inject a double dose to make up for forgotten doses. If you forget to inject a dose, tell your doctor immediately.
If you have any other questions about using this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, LITAK can cause side effects, although not everybody gets them.
Tell your doctor immediately if you experience any of the following symptoms or signs during treatment with LITAK or after:
- Any sign of infection (e.g., flu-like symptoms).
- Fever.
It cannot be excluded that a malignant disease (cancerous) may recur. This means that the risk of you developing a malignant tumor in the future is slightly higher than that of healthy individuals. This slightly increased risk may be due to hairy cell leukemia or the treatments used to treat the disease, including LITAK.
The following side effects may occur:
Very common side effects (may affect more than 1 in 10 patients)
- Infections.
- Fever.
- Low count of certain white blood cells (neutrophils and lymphocytes) and platelets in blood tests.
- Low red blood cell count, which can cause anemia, with symptoms such as fatigue and drowsiness.
- Decreased immune system function.
- Headache, dizziness.
- Abnormal breathing sounds, abnormal chest sounds, cough.
- Nausea, vomiting, constipation, and diarrhea.
- Rash, swelling, redness, and pain at the injection site, sweating.
Skin reactions are mainly mild or moderate and usually resolve within a few days.
- Fatigue, chills, decreased appetite.
- Weakness.
Common side effects (may affect up to 1 in 10 patients)
- Recurring malignant disease (cancerous).
- Low platelet count, which can cause unusual bleeding (e.g., nosebleeds or skin bleeding).
- Drowsiness, anxiety.
- Increased heart rate, abnormal heart sounds, low blood pressure, decreased blood flow to the heart muscle.
- Difficulty breathing, swelling of lung tissue due to infection, inflammation of the mouth and tongue.
- Abdominal pain in the presence of excess gas in the stomach or intestines, mainly mild increases in liver test values (bilirubin, transaminases), which will return to normal when treatment is finished.
- Rash with itching (urticaria), redness of the skin, and skin pain.
- Swelling of tissues (edema), discomfort, pain (muscle pain, joint pain, and bone pain).
Uncommon side effects (may affect up to 1 in 100 patients)
- Anemia caused by destruction of red blood cells.
- Drowsiness, numbness, and tingling of the skin, weakness, inactivity, peripheral nerve disorder, confusion, altered ability to coordinate movements.
- Eye inflammation.
- Sore throat.
- Vein inflammation.
- Severe weight loss.
Rare side effects (may affect up to 1 in 1,000 patients)
- Decreased liver function.
- Decreased kidney function.
- Complications caused by cancer treatment due to the destruction of cancer cells.
- Rejection reaction to blood transfusions.
- Increased count of certain white blood cells (eosinophils).
- Stroke.
- Speech and swallowing disorders.
- Heart failure.
- Abnormal heart rhythm.
- Inability of the heart to maintain adequate blood circulation.
- Intestinal obstruction.
- Severe allergic skin reaction (Stevens-Johnson syndrome or Lyell syndrome).
Very rare side effects (may affect up to 1 in 10,000 patients)
- Depression, epileptic seizure.
- Swelling of the eyelids.
- Blood clot in the lung.
- Inflammation of the gallbladder.
- Decreased function of organs due to high levels of a specific substance produced by the body (a glycoprotein).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing LITAK
Keep out of the sight and reach of children.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Do not use LITAK after the expiry date stated on the vial label and carton after EXP. The expiry date is the last day of the month indicated.
From a microbiological point of view, unless the opening of the product prevents the risk of microbiological contamination, the product should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user.
Do not use LITAK if you notice that the vial is damaged or if the solution is not clear or contains particles.
Disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
6. Contents of the pack and other information
Composition of LITAK
- The active substance is cladribine. Each ml of solution contains 2 mg of cladribine. Each vial contains 10 mg of cladribine in 5 ml of solution.
- The other ingredients are sodium chloride, sodium hydroxide (for pH adjustment), hydrochloric acid (for pH adjustment), and water for injections.
Appearance and pack contents
LITAK is marketed in glass vials containing 5 ml of clear and colorless injectable solution.
Pack of 1 or 5 vials. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Lipomed GmbH
Hegenheimer Strasse 2
D-79576 Weil/Rhein
Germany
You can request more information about this medicine by contacting the marketing authorization holder.
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
INSTRUCTIONS FOR INJECTION
This section contains information on how to administer an injection of LITAK. It is important that you do not attempt to administer the injection yourself unless your doctor or nurse has given you instructions. Your doctor will tell you how much LITAK you need and when you should inject it yourself. LITAK must be injected under the skin (subcutaneous injection). If you have any questions about administering the injection, ask your doctor or nurse.
LITAK is cytotoxic and should be handled with caution. When the patient is not self-administering LITAK, it is recommended to use disposable gloves and protective glasses when handling and administering LITAK. If LITAK comes into contact with the skin or eyes, rinse the affected area immediately with plenty of water. Pregnant women should avoid contact with LITAK.
What do I need for the injection?
To self-administer a subcutaneous injection, you will need:
- A vial of LITAK (or two vials if you need to inject more than 5 ml).
Do not use the vials if they are damaged or if the solution is not transparent or contains particles.
- A sterile syringe (e.g., a 10 ml LUER syringe).
- A sterile injection needle (e.g., 0.5 x 19 mm, 25 G x ¾ inch).
- Cotton wool / swab soaked in alcohol.
- A puncture-resistant container for the safe disposal of used syringes.
What should I do before injecting LITAK subcutaneously?
- Before injection, let the LITAK vial warm up to room temperature.
- Wash your hands carefully.
- Find a comfortable and well-lit place and put all the necessary material within reach.
How to prepare the injection?
Before injecting LITAK, you must do the following:
- Remove the protective red cap from the LITAK vial. Do not remove the rubber stopper from the vial. Clean the rubber stopper with a swab soaked in alcohol. Remove the syringe from the packaging without touching the tip. Remove the injection needle from the packaging and insert it firmly into the syringe tip. Remove the needle protection without touching the needle.
- Insert the needle through the rubber stopper of the vial and turn the vial and syringe upside down. Make sure the needle tip is submerged in the solution.
- Aspirate the exact volume of LITAK into the syringe by pulling back the plunger (your doctor will tell you how many ml of LITAK to inject).
- Remove the needle from the vial.
- Make sure there is no air in the syringe: hold the needle up and expel the air.
- Check that the volume of solution in the syringe is correct.
- Inject the solution immediately.
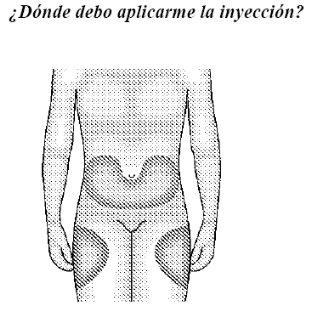
| This drawing shows the most convenient areas for injection: the upper part of the thighs and abdomen, except the area around the navel. If someone else is injecting you, they can also inject into the outer side of the arms or buttocks. |
|
|
- Gently pull back the plunger to ensure you have not punctured a blood vessel. If you see blood in the syringe, remove the needle and insert it into another area.
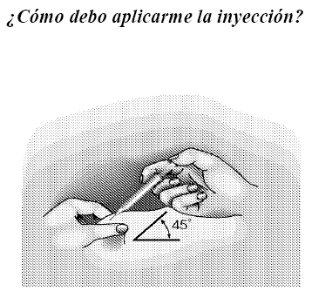
- Inject the liquid slowly and regularly over approximately 1 minute, keeping the skin pinched between your fingers.
- After injecting the liquid, remove the needle.
- Throw away the used syringe in a puncture-resistant container. Use a new syringe and injection needle for each injection. The vials are for single use. Return opened vials with unused solution to your doctor or pharmacist for proper disposal.
Disposal of used syringes
Throw away used syringes in a puncture-resistant container and keep it out of the reach and sight of children.
Dispose of the puncture-resistant container according to the instructions of your doctor, nurse, or pharmacist.
Do not throw away used syringes in your household trash.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LITAK 2 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 10 mg cladribine / 10 mlActive substance: cladribineManufacturer: Atnahs Pharma Netherlands Bv.Prescription requiredDosage form: INJECTABLEActive substance: cladribineManufacturer: Lipomed GmbhPrescription requiredDosage form: INJECTABLE INFUSION, 250 mgActive substance: nelarabineManufacturer: Sandoz Pharmaceuticals D.D.Prescription required
Online doctors for LITAK 2 mg/ml INJECTABLE SOLUTION
Discuss questions about LITAK 2 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions