
LIDOCAINE AGUETTANT 10 mg/mL INJECTABLE SOLUTION IN PRE-FILLED SYRINGE

How to use LIDOCAINE AGUETTANT 10 mg/mL INJECTABLE SOLUTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Lidocaína Aguettant 10mg/ml solution for injection in pre-filled syringe
Lidocaine hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Lidocaína Aguettant and what is it used for
- What you need to know before you use Lidocaína Aguettant
- How to use Lidocaína Aguettant
- Possible side effects
- Storage of Lidocaína Aguettant
- Contents of the pack and other information
1. What is Lidocaína Aguettant and what is it used for
Lidocaína Aguettant contains the active substance lidocaine hydrochloride.
Lidocaína Aguettant is a local anesthetic. It is used to numb parts of the body during surgical procedures. It prevents nerves from sending pain messages to the brain, and thus prevents you from feeling pain.
2. What you need to know before you use Lidocaína Aguettant
Do not use Lidocaína Aguettant:
- if you are allergic to lidocaine, local anesthetics of the amide type, or any other component of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you are given Lidocaína Aguettant:
- if you have epilepsy. Your doctor will closely monitor the symptoms.
- if you have kidney or liver disease.
- if you have a disease that causes muscle weakness (myasthenia gravis).
- if you have heart disorders, such as conduction disorders, slow heartbeats.
- if you have respiratory depression (difficulty breathing with slow and shallow breathing).
- if you are elderly or have a poor general state of health.
- if you have bleeding disorders or are being treated for them.
Additionally, your doctor knows that an injection of this medicine into inflamed tissue can cause an increase in the absorption of the active substance by the circulation and the effect of the active substance on your body will be weakened.
Your doctor will take into account that there is a greater risk of side effects on the nervous system if this medicine is administered in the head and neck region.
Children and adolescents
Lidocaína Aguettant 10 mg/ml must not be used in children under 2 years of age.
Using Lidocaína Aguettant with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Lidocaína Aguettant may affect other medicines or be affected by them.
In particular, tell your doctor if you are taking any of the following:
- medicines used to treat high blood pressure, such as diuretics;
- medicines used to treat heart disorders, including irregular heartbeats, such as beta-blockers (e.g., metoprolol, propranolol) or calcium antagonists (e.g., amiodarone);
- medicines that constrict blood vessels (vasoconstrictors, e.g., epinephrine, norepinephrine);
- medicines used to relax muscles during general anesthesia (e.g., suxamethonium);
- sleeping pills and medicines that reduce the level of consciousness (sedatives);
- medicines that increase the risk of having epileptic seizures and convulsions (e.g., tramadol, bupropion);
- medicines that reduce the risk of having epileptic seizures and convulsions (e.g., diazepam);
- cimetidine, a medicine used to treat heartburn;
- antiviral medicines (e.g., ritonavir), macrolide antibiotics (e.g., erythromycin), or antifungal medicines (e.g., ketoconazole, itraconazole);
- ciprofloxacin (antibiotics);
- medicines used to treat epilepsy (phenobarbital, phenytoin, carbamazepine, or primidone);
- fluvoxamine, a medicine used in the treatment of mental illness;
- medicines used to reduce intraocular pressure (e.g., acetazolamide);
- other anesthetics, including local anesthetics.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. Your doctor will then decide whether you should be given this medicine.
Pregnancy
Your doctor will only give you this medicine while you are pregnant if he considers it necessary. The dose should be as low as possible.
Breastfeeding
Lidocaine passes into human breast milk in small amounts. The use of lidocaine at the recommended doses is unlikely to affect the breastfed infant. Therefore, breastfeeding can continue during the use of lidocaine.
Driving and using machines
Lidocaína Aguettant may affect your ability to drive or use machines. Ask your doctor when it would be safe to drive or use machines.
Lidocaína Aguettant contains sodium
This medicine contains 32 mg of sodium (main component of table salt/kitchen salt) in each syringe. This is equivalent to 1.6% of the maximum daily intake of 2 mg of sodium recommended by the WHO for an adult.
3. How to use Lidocaína Aguettant
Administration will be carried out by a healthcare professional with the appropriate training and experience.
Your doctor will decide what dose is appropriate for your particular case, according to your age and state of health, as well as the injection site, the method used, and your response to the injection.
Use in children and adolescents
Lidocaína Aguettant 10 mg/ml must not be used in children under 2 years of age.
Method of administration
Lidocaína Aguettant will be administered as an infiltration injection (via intradermal, subcutaneous, or submucosal route) in the areas surrounding the peripheral nerves.
If you use more Lidocaína Aguettant than you should
Since this medicine is administered by a healthcare professional with training, it is unlikely that you will be given too much Lidocaína Aguettant.
If you develop symptoms of overdose or not, it depends on the level of medicine present in your blood. The more lidocaine there is in your blood and the faster it is administered, the more likely you are to experience symptoms of an overdose with greater frequency and intensity.
A small overdose mainly affects your central nervous system. The adverse effects that occur will disappear in most cases after the administration of lidocaine is interrupted.
Regardless of this, if you think you have received too much of the medicine, or start to experience dizziness or drowsiness, numbness of the tongue, ringing in the ears, vomiting, or chills, you should inform the person who administered the injection immediately. The doctor will know how to control these symptoms and will administer any necessary treatment.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects can be serious. Get medical help immediately if you experience an allergic reaction that causes:
- swelling of hands, feet, face, lips, mouth, tongue, or throat
- difficulty breathing
- itchy rash
- fever
- drop in blood pressure and shock.
These side effects are rare (may affect up to 1 in 1000 people).
Other side effects may include:
Very common (may affect more than 1 in 10 people)
- nausea.
Common (may affect up to 1 in 10 people)
- tingling, numbness, burning, prickling, or feeling of pins and needles (paresthesia)
- loss of consciousness
- pain or chills due to the injections
- slow heartbeats
- low blood pressure or high blood pressure
- vomiting.
Rare (may affect up to 1 in 1000 people)
- changes in sensations or muscle weakness (neuropathy)
- seizures
- partial paralysis
- headache accompanied by a buzzing or crackling sound in the ears (tinnitus) and an abnormal intolerance to light (photophobia)
- hearing loss (deafness)
- nerve damage
- drooping of the eyelids combined with constriction of the pupils and, occasionally, decreased sweating (Horner's syndrome). This occurs after application in the head/neck region.
- asymmetric sweating and flushing of the upper chest, neck, or face (harlequin syndrome)
- irregular heartbeats
- heart arrest
- double vision
- slow or interrupted breathing
- rash or hives.
Frequency not known (cannot be estimated from the available data)
- bluish discoloration of the skin, headaches, difficulty breathing, and fatigue due to abnormal amounts of methemoglobin (a form of hemoglobin that has a reduced ability to bind oxygen) in the blood (methemoglobinemia)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Lidocaína Aguettant
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label, blister, and carton of the syringe. The expiry date is the last day of the month stated.
Keep the pre-filled syringe in the closed blister until use. Do not freeze.
Once opened, the medicine must be used immediately.
Do not use this medicine if you notice visible signs of deterioration.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Lidocaína Aguettant 10mg/ml solution for injection in pre-filled syringe
The active substance is lidocaine hydrochloride.
- Each ml of the solution for injection contains 10 mg of lidocaine hydrochloride (as lidocaine hydrochloride monohydrate).
- Each pre-filled syringe of 5 ml contains 50 mg of lidocaine hydrochloride (as lidocaine hydrochloride monohydrate).
- Each pre-filled syringe of 10 ml contains 100 mg of lidocaine hydrochloride (as lidocaine hydrochloride monohydrate).
The other ingredients are: sodium chloride, sodium hydroxide, concentrated hydrochloric acid (for pH adjustment), water for injections
Appearance and packaging
Lidocaína Aguettant 10 mg/ml solution for injection in pre-filled syringe is a clear, colorless solution for injection (injection). Lidocaína Aguettant is available in a 5 or 10 ml pre-filled syringe, packaged individually in a transparent blister. Carton box of 1 or 10 pre-filled syringes. Not all pack sizes may be marketed.
Marketing authorization holder
Laboratoire Aguettant
1 rue Alexander Fleming
69007 Lyon
France
Manufacturer
Laboratoire Aguettant
1 rue Alexander Fleming
69007 Lyon
France
Laboratoire Aguettant
Lieu-Dit Chantecaille
07340 Champagne
France
Local representative:
Aguettant Ibérica S.L.
Parc Científic de Barcelona
Baldiri Reixac, 4-8 (Torre I)
08028 Barcelona
Date of last revision of this leaflet: 06/2024.
------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Prepare the pre-filled syringe carefully as follows
The pre-filled syringe is for single patient use only. Discard the pre-filled syringe after use. Do not reuse it.
The contents of an unopened or undamaged pack are sterile and should not be opened until the moment of use.
The medicine should be inspected visually for particles or color changes before administration. The solution should only be used if it is clear, colorless, and no particles or precipitates are observed.
The medicine should not be used if the security seal of the syringe is broken.
The outer surface of the pre-filled syringe is sterile until the blister is opened.
When handled using an aseptic method, this medicine can be placed in a sterile area.
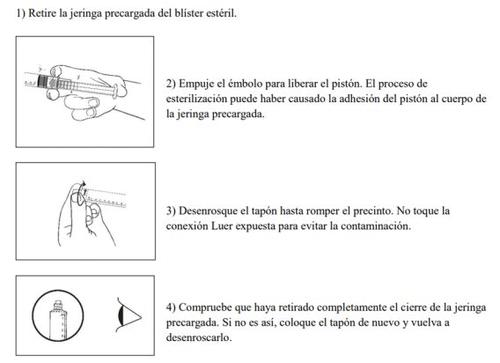

- Connect the pre-filled syringe to the access device or needle. Slowly push the plunger to inject the required volume.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LIDOCAINE AGUETTANT 10 mg/mL INJECTABLE SOLUTION IN PRE-FILLED SYRINGEDosage form: DRESSING, 700 mgActive substance: lidocaineManufacturer: Grünenthal Pharma S.A.Prescription requiredDosage form: GEL/PASTE/ORAL LIQUID, 20 mg/gActive substance: lidocaineManufacturer: Chemische Fabrik Kreussler & Co GmbhPrescription not requiredDosage form: GEL, 20 mg/mlActive substance: lidocaineManufacturer: Farco-Pharma GmbhPrescription required
Online doctors for LIDOCAINE AGUETTANT 10 mg/mL INJECTABLE SOLUTION IN PRE-FILLED SYRINGE
Discuss questions about LIDOCAINE AGUETTANT 10 mg/mL INJECTABLE SOLUTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















