
LAINEMA 14/3 g/100 ml RECTAL SOLUTION


How to use LAINEMA 14/3 g/100 ml RECTAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
LAINEMA 14/3 g/100 ml rectal solution EFG
Sodium dihydrogen phosphate (monohydrate)/Disodium hydrogen phosphate (dodecahydrate)
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you, do not pass it on to others, it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the pack:
- What LAINEMA 14/3 g/100 ml rectal solution EFG is and what it is used for
- Before using LAINEMA 14/3 g/100 ml rectal solution EFG
- How to use LAINEMA 14/3 g/100 ml rectal solution EFG
- Possible side effects
- Storage of LAINEMA 14/3 g/100 ml rectal solution EFG
- Further information
1. What LAINEMA 14/3 g/100 ml rectal solution EFG is and what it is used for
This medicine belongs to the pharmacotherapeutic group of rectal laxatives.
This medicine is indicated in cases where intestinal evacuation is necessary, such as: pre- and post-surgery, childbirth and post-childbirth, before rectoscopy, sigmoidoscopy, and colonoscopy (techniques for exploring the large intestine), before radiological examinations, fecal impaction (accumulation of hardened feces in the rectum).
2. Before using LAINEMA 14/3 g/100 ml rectal solution EFG
Do not use LAINEMA 14/3 g/100 ml rectal solution EFG
- if you are allergic (hypersensitive) to the active substances or to any of the other components of this medicine
- in children under 2 years of age
- if there is suspicion of intestinal obstruction (inability to eliminate feces and gases)
- if you have megacolon (dilation of the colon), ileostomy (artificial anus), anorectal stenosis (narrowing of the rectum), imperforate anus (congenital absence or obstruction of the anal orifice) or paralytic ileus (paralysis of the intestine)
- if you have severe or moderate kidney problems
- if you have congestive heart failure (severe heart problem)
- if you have symptoms of appendicitis or intestinal perforation
- if you have undiagnosed rectal bleeding
- if you have uncontrolled high blood pressure
- if you are dehydrated and, in general, in all cases where the distribution of LAINEMA's content in the body may be increased or its elimination decreased.
In case of doubt about any of these situations or your particular situation, consult your doctor.
Be careful with LAINEMA 14/3 g/100 ml rectal solution EFG
If after 10 minutes of administering the enema you do not achieve evacuation (evacuation occurs approximately 5 minutes after administration), it is recommended that you consult your doctor so that they can perform the necessary tests to minimize the risk of severe hyperphosphatemia.
- If you are elderly, weak, suffer from ascites (fluid accumulation in the abdomen), anal ulcers or fissures, or have kidney or heart disease.
- If you have had a colostomy (major colon surgery), you should consult your doctor.
- If you have pre-existing electrolyte imbalances (alteration of mineral salt levels in the body) as hypocalcemia (decreased calcium levels in the blood), hypokalemia (decreased potassium levels in the blood), hyperphosphatemia (increased phosphorus levels in the blood), hypernatremia (increased sodium levels in the blood), and acidosis may occur.
- In case of suspected electrolyte disorders, your doctor will perform a blood test to determine the levels of these substances before administering LAINEMA.
- If you have nausea, vomiting, or abdominal pain, follow your doctor's instructions.
- Repeated and prolonged use of LAINEMA is not recommended, as it may cause habituation. Do not use if symptoms worsen or persist without consulting a doctor.
- LAINEMA should be administered following the instructions for use and handling. You should interrupt administration if you encounter resistance, as forcing administration can cause injuries.
- If you bleed from the rectum after administering the enema, do not administer more enemas and consult your doctor immediately.
- Consult your doctor if signs of irritation occur.
This medicine should not be used as a habitual treatment for constipation.
Using other medicines
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription, homeopathic medicines, herbal remedies, and other health-related products, as it may be necessary to interrupt treatment or adjust the dose of one of them.
It is especially important that your doctor knows if you are taking medicines for high blood pressure or angina pectoris (calcium channel blockers), medicines to empty the bladder (diuretics), medicines for certain behavioral disorders (lithium), or other drugs that could modify the balance of water or electrolytes (minerals) in the blood.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medicine.
Do not use this medicine during pregnancy or breastfeeding without consulting your doctor.
If you are breastfeeding, you should express and discard the milk produced during the 24 hours following administration of LAINEMA.
Driving and using machines
This medicine does not affect your ability to drive or use machines.
Important information about some of the components of LAINEMA 14/3 g/100 ml rectal solution EFG
It may cause allergic reactions (possibly delayed) and, exceptionally, bronchospasm, as it contains methyl parahydroxybenzoate, sodium salt (E-219).
3. How to use LAINEMA 14/3 g/100 ml rectal solution EFG
Follow exactly the administration instructions of the medicine indicated by your doctor. Consult your doctor or pharmacist if you have doubts.
This is a rectal medicine. It should be applied at room temperature, without the need to heat it.
For self-administration of this medicine, it is recommended that the patient be reclined on their left side with both legs bent over the chest (Figure 1) or reclined with the left leg extended and the right leg bent over the chest (Figure 2).

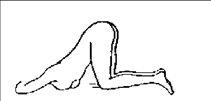
When this medicine is going to be administered to the patient by another person, the recommended positions may be either those described for self-administration or the one shown in Figure 3.

Press the cap to one side. This breaks the seal. Once the seal is broken, pull the cap upwards and the pre-lubricated cannula is free for insertion.
In the indicated positions, carefully insert the cannula into the rectum to avoid damaging the wall, and press the container in a smooth and continuous manner until the required amount of liquid is inserted. It is advisable for the patient to maintain this position until they feel strong urges to defecate. Generally, 2 to 5 minutes are sufficient to achieve the desired effect. If the product is not expelled after this time, see section 4: “Possible side effects”.
In general, the following dose is recommended:
Children
-Infants and children under 2 years: LAINEMA should not be administered to infants and children under 2 years of age, its use is contraindicated.
-Children from 2 to 15 years:The recommended dose is a single dose of 5 ml/kg, or up to a maximum of 140 ml. The maximum duration of treatment will be one enema per day, for no more than 6 consecutive days.
Adults
The recommended dose is a single enema of 140 ml or 250 ml. It can be administered once a day, for a maximum of 6 consecutive days.
Over 65 years:The recommended dosage is the same as for adults.
Patient with liver problems: In this case, no dose adjustment is necessary.
Patient with kidney problems:This medicine should not be administered to patients with severe or moderate kidney problems.
It will be administered with caution to patients with mild kidney problems and only under medical prescription.
If you use more LAINEMA 14/3 g/100 ml rectal solution EFG than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use LAINEMA 14/3 g/100 ml rectal solution EFG
Do not use a double dose to make up for forgotten doses.
If you have any other doubts about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, LAINEMA can cause side effects, although not everybody gets them.
In very rare cases, cases of tetany (painful muscle spasms) with severe hypocalcemia (decreased calcium in the blood) and hyperphosphatemia (increased phosphorus in the blood) may occur. Serious cases of hyperphosphatemia associated with the administration of laxatives with high phosphate content have been reported. Patients with risk factors for developing hyperphosphatemia should, therefore, be monitored through analytical tests (see section “Be careful with LAINEMA 14/3 g/100 ml rectal solution EFG”)
Patients who very rarely develop severe hyperphosphatemia may present irritability, hypotension (low blood pressure), muscle cramps, cyanosis (bluish skin discoloration), tetany, tachycardia (increased heart rate), convulsions, obtundation, fatigue, weakness, or potentially a comatose state.
The following are the known side effects of LAINEMA, listed according to their frequency:
Very rare side effects(affecting less than 1 in 10,000 patients):
-tetany
-hypocalcemia
-severe hyperphosphatemia
-blisters
-burning sensation
-itching
-rectal irritation
-pain
Very common side effects(affecting at least 1 in 10 patients)
- Transient hyperphosphatemia
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
5. Storage of LAINEMA 14/3 g/100 ml rectal solution EFG
No special storage conditions are required, however, in rare cases, some inorganic flocculations may appear in the solution that do not affect the integrity of the preparation.
Keep out of the reach and sight of children.
Do not use LAINEMA after the expiration date stated on the packaging after EXP. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. FURTHER INFORMATION
Composition of LAINEMA 14/3 g/100 ml rectal solution EFG
The active substances per milliliter are: sodium dihydrogen phosphate (monohydrate) 139 mg, disodium hydrogen phosphate (dodecahydrate) 32 mg. The other components (excipients) are: methyl parahydroxybenzoate, sodium salt (E-219), and purified water.
Appearance of the product and packaging content
This medicine belongs to a group of medicines called rectal laxatives.
It is presented as a rectal solution, in low-density polyethylene bottles of 80, 140, and 250 ml, and a rectal applicator formed by a high-density polyethylene cap, a pre-lubricated EBA (ethyl acrylate copolymer) cannula, a seal, and a high-density polyethylene cap.
Marketing authorization holder and manufacturer
LAINCO, S.A. Avda. Bizet, 8-12 - 08191 Rubí (Barcelona)
This leaflet was approved in October 2017
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LAINEMA 14/3 g/100 ml RECTAL SOLUTIONDosage form: RECTAL LIQUID, 3.2 g / 13.9 gActive substance: sodium phosphateManufacturer: Casen Recordati S.L.Prescription requiredDosage form: RECTAL LIQUID, 6.14 ml glycerolActive substance: glycerolManufacturer: Casen Recordati S.L.Prescription not requiredDosage form: RECTAL LIQUID, 450 mg / 45 mgActive substance: sodium lauryl sulfoacetate, incl. combinationsManufacturer: Lainco S.A.Prescription not required
Online doctors for LAINEMA 14/3 g/100 ml RECTAL SOLUTION
Discuss questions about LAINEMA 14/3 g/100 ml RECTAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















