IXCHIQ POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use IXCHIQ POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
IXCHIQ Powder and Solvent for Solution for Injection
Chikungunya Vaccine (Live)
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start receiving this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is IXCHIQ and what is it used for
- What you need to know before you receive IXCHIQ
- How IXCHIQ is administered
- Possible side effects
- Storage of IXCHIQ
- Contents of the pack and further information
1. What is IXCHIQ and what is it used for
IXCHIQ is a vaccine that helps protect adults 18 years of age and older against disease caused by the chikungunya virus (CHIKV).
Chikungunya is a disease caused by the chikungunya virus (CHIKV), which is found in subtropical regions of the Americas, Africa, Southeast Asia, India, and the Pacific region. CHIKV is transmitted to humans through the bite of an infected mosquito. Most people infected with CHIKV develop sudden fever and intense pain in several joints. Other symptoms may include headache, muscle pain, joint swelling, or skin rash. These symptoms usually disappear within 7 to 10 days but can last for months or years.
Consult your doctor, pharmacist, or nurse before deciding whether you should receive this vaccine.
How the vaccine works
IXCHIQ works by teaching the immune system (the body's natural defenses) to defend against CHIKV. The vaccine contains a weakened form of the virus that has been weakened in the laboratory so that it cannot multiply. When the body encounters this weakened version of the virus, the immune system recognizes it and produces antibodies to attack it. When a vaccinated person later comes into contact with the virus, their immune system will recognize it and be prepared to defend the body. This helps prevent illness.
2. What you need to know before you receive IXCHIQ
The vaccine must not be administered:
- If you are allergic to the active substance or any of the other components of this vaccine (listed in section 6).
- If your immune system has a reduced ability to fight infections and other diseases (immunodeficiency) or if you have a weakened immune system (immunocompromised) due to a disease or medication (such as cancer and chemotherapy, inherited immune problems, prolonged use of medications that weaken the immune system, such as corticosteroids or immunosuppressants, or HIV infection).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before receiving IXCHIQ:
- If you have had a severe allergic reaction after receiving any other injectable vaccine.
- If you have ever fainted after an injection with a needle or if needles or injections cause you anxiety.
- If you have a bleeding problem, bruise easily, or are taking anticoagulant medications (to prevent blood clots).
- If you have had a recent fever (body temperature above 38°C). However, you may be vaccinated if you have a mild fever or an upper respiratory tract infection, such as a cold.
Do not donate blood for at least 4 weeks after being vaccinated with IXCHIQ.
If you are unsure if any of the above applies to you, consult your doctor, pharmacist, or nurse before receiving the vaccine.
It is possible that IXCHIQ may not completely protect all people who receive the vaccine. IXCHIQ does not protect against other mosquito-borne diseases.
You must protect yourself from mosquito bites even after receiving the IXCHIQ vaccine. If you travel to countries where the chikungunya virus is still present, use insect repellent, wear long-sleeved pants and shirts, and stay in air-conditioned or screened areas.
Children and adolescents
IXCHIQ has not been fully tested in young people under 18 years of age. It must not be used in this age group.
Other medicines and IXCHIQ
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before receiving this vaccine.
IXCHIQ has not been studied in pregnant women or breastfeeding mothers.
Driving and using machines
Some of the side effects of IXCHIQ (see section 4) may temporarily affect your ability to drive or use machines. Do not drive or use machines if you do not feel well after receiving the vaccine. Wait until the effects of the vaccine have disappeared before driving or using machines.
IXCHIQ contains potassium and sodium
This vaccine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
This vaccine contains less than 1 mmol of potassium (39 mg) per dose; this is essentially "potassium-free".
3. How IXCHIQ is administered
IXCHIQ is administered by a doctor, pharmacist, or nurse as a single 0.5 ml injection into the muscle of the upper arm.
If you have any further questions on the use of this vaccine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Seek urgent medical attention if you experience symptoms of a severe allergic reaction. Such reactions may include a combination of any of the following symptoms:
- difficulty breathing
- hoarseness or wheezing
- hives or skin rash
- swelling of lips, face, or throat
- dizziness
- weakness
- rapid heartbeat
The following side effects may also occur after receiving this vaccine.
Very common (may affect more than 1 in 10 people):
- headache
- nausea
- fatigue
- muscle pain
- joint pain
- fever
- pain, redness, swelling, or itching at the injection site
- low white blood cell count
- high levels of liver enzymes, measured in blood tests
Common (may affect up to 1 in 10 people):
- swollen lymph nodes
- skin rash
- chills
- back pain
- diarrhea
- vomiting
Uncommon (may affect up to 1 in 100 people):
- dizziness
- tingling, burning, or prickling sensation, usually in the hands, arms, legs, or feet
- eye pain
- ringing or buzzing in the ears
- difficulty breathing
- excessive sweating
- physical weakness
- swelling in the lower legs or hands
Rare (may affect up to 1 in 1,000 people):
- low levels of water and sodium in the blood
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that the side effects are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of IXCHIQ
Keep this medicine out of the sight and reach of children.
Your doctor, pharmacist, or nurse is responsible for storing this medicine and disposing of any unused product correctly. This information is intended for healthcare professionals.
Do not use this medicine after the expiry date which is stated on the carton, vial, and syringe after "EXP". The expiry date is the last day of the month shown.
Store in a refrigerator (2°C to 8°C). Do not freeze.
Store in the original carton to protect from light.
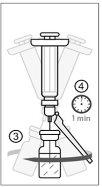
The stability of the reconstituted vaccine has been demonstrated for 2 hours when stored refrigerated at a temperature between 2°C and 8°C or at room temperature (15°C to 25°C).
After this time, the reconstituted vaccine must be discarded.
From a microbiological point of view, once opened, the vaccine should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user.
Vaccines should not be disposed of via wastewater or household waste. Your doctor or nurse will dispose of this vaccine. This will help protect the environment.
6. Contents of the pack and further information
Composition of IXCHIQ
After reconstitution, 1 dose of 0.5 ml contains the Δ5nsP3 strain of the chikungunya virus (live, attenuated)*, not less than 3.0 log10 TCID50**.
*Produced in Vero cells
**Tissue culture infectious dose at 50%
This product contains genetically modified organisms (GMOs).
The other ingredients are:
Powder: sucrose, D-sorbitol, L-methionine, trisodium citrate dihydrate, magnesium chloride, dipotassium hydrogen phosphate, and recombinant human serum albumin of fungal production (Saccharomyces cerevisiae).
Solvent: sterile water for injections
See section 2 "IXCHIQ contains potassium and sodium".
Appearance and pack contents
IXCHIQ is a powder and solvent for solution for injection. The powder is white to slightly yellowish in color. The solution is a clear, colorless liquid.
Each pack of IXCHIQ contains:
- 1 vial containing the IXCHIQ component as a powder for 1 dose in the form of a white to slightly yellowish powder.
- 1 pre-filled syringe containing the solvent for 1 dose of the water component in the form of a clear solution.
The contents of the two components (vial and syringe) must be mixed before vaccination to obtain a 0.5 ml dose.
Marketing authorization holder and manufacturer
Valneva Austria GmbH
Campus Vienna Biocenter 3
1030 Vienna
Austria
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
<--------------------------------------------------------------------------------------------------------------------------->
This information is intended only for healthcare professionals:
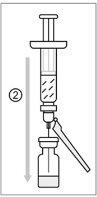
The vaccine must be reconstituted only with the supplied solvent before administration.
For reconstitution of the vaccine, a needle (22-25G) of adequate length should be used; preferably, at least 40 mm (1 1/2") in length.
The syringe is for single use only.
The reconstituted vaccine is a clear, colorless to slightly yellowish liquid solution. Before administering the vaccine, it is recommended to visually inspect for the presence of particles and unusual coloration, as far as the solution and container permit. If any of these conditions exist, do not administer the vaccine.
| Figure 1
|
| Figure 2
|
| Figure 3
|
| Figure 4
|
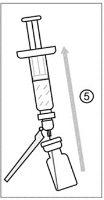
After reconstitution, administer IXCHIQ by intramuscular injection within 2 hours. If not used within 2 hours, discard the reconstituted vaccine.
Disposal
This product contains genetically modified organisms (GMOs). Disposal of unused vaccine and all materials that have come into contact with it should be done according to local guidelines for pharmaceutical waste. Any spills should be cleaned up immediately and disinfected according to local policies. Dispose of the used needle and syringe in a puncture-resistant container, such as a container that can be closed and is resistant to punctures.
- Country of registration
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IXCHIQ POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 60 micrograms/dose + 60 micrograms/doseActive substance: respiratory syncytial virus vaccinesManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 0.5 mLActive substance: respiratory syncytial virus vaccinesManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 50 µgActive substance: respiratory syncytial virus vaccinesManufacturer: Moderna Biotech Spain S.L.Prescription required
Online doctors for IXCHIQ POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about IXCHIQ POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions