
INSTILLIDO 20 mg/ml GEL

How to use INSTILLIDO 20 mg/ml GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Instillido 20mg/ml gel
lidocaine hydrochloride (as lidocaine hydrochloride monohydrate)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Instillido and what is it used for
- What you need to know before you use Instillido
- How to use Instillido
- Possible side effects
- Storing Instillido
- Contents of the pack and further information
1. What is Instillido and what is it used for
Instillido is a sterile gel that contains the active ingredient lidocaine hydrochloride (as lidocaine hydrochloride monohydrate). Throughout this leaflet, it will be referred to as “lidocaine”.
Lidocaine has a local anesthetic effect and is used to numb the parts of the body where the gel is applied. It prevents nerves from sending pain messages to the brain and, therefore, makes you not feel pain.
Due to its local anesthetic effectand lubricating propertiesfor catheters, endoscopes, or other medical instruments, this medicine is designed to relieve discomfort and facilitate the process during certain types of examinations or procedures. This medicine is used
- for instillation (introduction) into the urethra before inserting or replacing a catheter and for cystoscopy, when a doctor inserts a tube through the urethra to visualize the bladder.
- for proctoscopy/rectoscopy (medical procedures in which an instrument called an endoscope is used to examine the anal cavity or rectum). During this procedure, this medicine is instilled into the anal/rectal cavity and/or the instrument is lubricated with this medicine before insertion.
Due to its anesthetic effect, this medicine is also used
- to help relieve pain from inflammation of the urinary bladder.
This medicine is indicated for adults, adolescents (over 12 years of age), and children from 2 to 12 years.
Your doctor will explain for which specific procedure or condition this medicine is used.
The application will normally be performed by a doctor, but it can also be administered by you or your caregiver, for example, for self-catheterization (see section 3 “How to use Instillido”).
2. What you need to know before you use Instillido
Do not use Instillido
- if you are allergic to lidocaine hydrochloride or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to other local anesthetics (of the amide type).
- in children under 2 years of age.
Warnings and precautions
Consult your doctor or pharmacist before starting to use this medicine.
Before using this medicine, your doctor needs to know if you have or have had any of the following diseases:
- if you have any wounds, injuries to the mucous membranes, or an ulcer/inflammation in or around the proposed application site.
- if your liver or kidney function is impaired, if you are severely ill, are in a state of weakness, or have sepsis (“blood poisoning”). Your doctor may reduce the dose of this medicine.
- if you have a slow heart rate, heart dysfunction, or respiratory tract problems (problems with the airways).
- if you have a weak heart (heart failure) or conduction disorders (atrioventricular block).
- if you are suffering from medical shock (shock).
- if you are prone to seizures (attacks) or suffer from epilepsy.
- if you suffer from a certain muscular disease (myasthenia gravis).
- if you suffer from a rare hereditary disease that affects the blood called “glucose-6-phosphate dehydrogenase deficiency”.
- if you have a problem with blood pigment levels called “methemoglobinemia”.
- if you suffer from porphyria (a blood disorder).
- if you are being treated with certain medications for heart rhythm disorders, known as class III antiarrhythmics (e.g., amiodarone), as they may increase the effects on the heart. See also the section “Other medicines and Instillido”.
Also, inform your doctor if you frequently use other medications that contain lidocaine and/or in high doses, as this may cause serious side effects.
If this medicine is introduced into the urethra and a large amount of gel reaches the bladder or if the urethra is ulcerated/inflamed, this may generally lead to an increased absorption of lidocaine through the mucous membranes, particularly in children and elderly patients, resulting in serious side effects (see also section 3, “If you have been given / if you use more Instillido than you should”).
Other medicines and Instillido
Tell your doctor or pharmacist if you are taking/using, have recently taken/used, or might take/use any other medicines. Lidocaine may affect or be affected by other medicines.
You must tell your doctor if you are taking/using any of the following medicines:
- antiarrhythmics: medicines for treating irregular heartbeats (e.g., mexiletine, amiodarone).
- calcium antagonists: medicines taken for heart conditions or high blood pressure (e.g., diltiazem, verapamil).
- beta-blockers(e.g., propranolol, metoprolol): for treating high blood pressure or angina pectoris.
These medicines may have a greater effect on the heart.
- other medicines that contain lidocaineor some other local anesthetics(of the amide type), as they may potentiate their respective effects in an unpredictable manner.
- cimetidinefor treating hyperacidity and stomach and duodenal ulcers. Using this medicine at the same time may increase the risk of side effects.
- fluvoxaminefor treating depression.
- erythromycin(antibiotic).
- protease inhibitors used to treat HIV (e.g., ritonavir).
- medicines used to treat infections, called sulfonamidesand nitrofurantoin.
- medicines used to treat epilepsy, called phenytoinand phenobarbital.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
During pregnancy and breastfeeding, this medicine should only be used after your doctor has carefully considered the benefits and risks.
Driving and using machines
The effects on the ability to drive and use machines are unlikely, but cannot be completely ruled out in cases of greater individual sensitivity. If you feel drowsy, dizzy, or have vision disturbances, do not drive or use any tools or machinery.
3. How to use Instillido
Administration is usually performed by a doctor with the appropriate training and relevant experience.
If you are treating yourself, such as for self-catheterization (insertion of a small plastic tube (catheter) into your own urethra), always use this medicine exactly as your doctor has told you and follow the instructions on how to apply the gel. If in doubt, ask your doctor.
This medicine starts to work between 5 and 15 minutes after application. The effect usually lasts between 20 and 30 minutes.
Dose
Your doctor will decide the most suitable dose for your particular case based on your age and state of health, as well as the application site, method used, and your response.
The recommended dose is:
Adults
For instillation (introduction) into the urethra
Male patients
For adequate pain relief, 20 ml of gel is usually needed.
When anesthesia is especially important, e.g., during catheterization or cystoscopy, your doctor may instill a larger amount of gel (up to 40 ml).
For catheterization, small volumes (5-10 ml) are usually sufficient for lubrication.
Female patients
The doctor will adapt the amount of gel to be introduced to the individual anatomical conditions of the urethra. Usually, 5 to 10 ml of gel is instilled in small portions to fill the entire urethra.
For relief of pain from inflammation of the urinary bladder
Usually, 10 to 20 ml of gel is needed for adequate pain relief.
The doctor decides on the frequency and duration of use based on your condition and symptoms. The maximum dose is: 20 ml of gel once a day.
Proctoscopy/rectoscopy
For adequate pain relief, the doctor will usually instill 10 to 20 ml of gel into the anal/rectal cavity and apply a small amount to lubricate the endoscope.
Maximum dose
The dose depends on the application site. A safe dose for use in the urethra and bladder in adults is 40 ml of gel (approximately 800 mg of lidocaine hydrochloride). The maximum recommended daily dose is approximately 800 mg of lidocaine hydrochloride.
Special populations
Your doctor may decide to reduce the dose if you are elderly, severely ill, are in a state of weakness, have liver or kidney problems, or have sepsis (“blood poisoning”). Do not exceed a maximum dose of 2.9 mg/kg body weight of lidocaine hydrochloride.
Use in children and adolescents
Children < 2 years of age
This medicine must not be used in children under 2 years of age.
Children (2 - 12 years) and adolescents (over 12 years of age)
The doctor will determine the dose based on the child's age, weight, and physical condition.
Do not exceed a maximum dose of 2.9 mg/kg body weight of lidocaine hydrochloride in children (from 2 to 12 years).
Method of administration
Pre-filled graduated syringes are available with 6 ml or 11 ml of gel. Your doctor will choose the appropriate size based on the amount you need.
Each graduation on the syringe corresponds to approximately 1 ml of gel (20.1 mg of lidocaine hydrochloride).
For (self-)catheterization (urethral route)
Follow these instructions carefully:
- Wash your hands. Clean and disinfect the genital area.
- When you are ready to use it, open the blister.
- Before removing the cap from the tip of the syringe, press the plunger to eliminate any resistance that may be present. This helps ensure that the syringe empties easily and uniformly.
?Figure 1?
- Remove the cap from the tip of the syringe. The syringe is now ready for use. ?Figure 2?
- Insert the nozzle into the urethral opening and press the plunger slowly and evenly to push the gel into the urethra. ?Figure 3?
- Wait a few minutes after instilling the gel for the anesthetic to take full effect. The complete anesthetic effect will occur between 5 and 15 minutes after complete instillation.
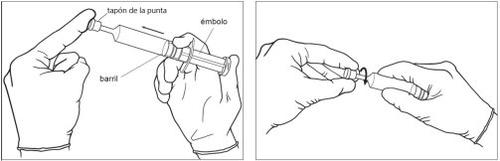 Figure 1:Figure 2:
Figure 1:Figure 2:
Figure 3:
Male patients: Female patients:
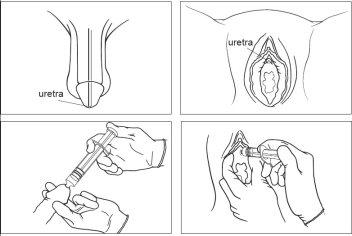
For cystoscopy
Your doctor will administer this medicine into the urethra and/or apply it to the endoscope.
For proctoscopy/rectoscopy
Your doctor will administer this medicine into the anal/rectal cavity and/or apply it to the endoscope.
The syringe is for single use.Use immediately after opening the blister. The syringe and any unused gel should be discarded.
Duration of treatment
For examinations/procedures, this medicine is usually used only for one or two doses or for short-term treatment.
If you have been prescribed this medicine for self-administration, your doctor will decide how long you should continue using this medicine based on your condition.
If you have been given / if you use more Instillido than you should
Administration of Instillido by a doctor
Since this medicine is usually administered by a qualified doctor, it is unlikely that you will be given too much of this medicine. Nevertheless, if you think you have been given too much medicine or start to experience the symptoms of overdose listed below, you should inform the person administering this medicine immediately. Your doctor will know how to treat these symptoms and will give you the necessary treatment.
Administration of Instillido by yourself or by a caregiver for self-catheterization
Whether or not you have symptoms of overdose depends on the level of this medicine present in your blood. The more lidocaine in your blood, the more severe the symptoms of overdose you may experience. Normally, only small amounts of the active ingredient lidocaine are absorbed into the blood from this medicine. Too much lidocaine can be absorbed if the surface to be treated is damaged.
The first symptoms of overdose are, for example:
hearing, vision, speech, and/or movement coordination problems, yawning, restlessness, dizziness, nausea, and vomiting.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicological Information Service, telephone 91 562 04 20, indicating the medicine and the amount ingested, even if you do not have symptoms.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Lidocaine is generally well tolerated as long as the medicine is used as indicated in the section “3. How to use Instillido” and the necessary precautions are taken (see section “2. What you need to know before you use Instillido”).
Some side effects can be serious. Seek immediate medical attention if you have an allergic reaction (hypersensitivity) that causes:
- swelling of the hands, feet, face, lips, mouth, tongue, or throat
- difficulty breathing
- difficulty breathing due to narrowing of the airways (bronchospasm)
- skin problems, such as itching or rashes
- hives
- increased blood pressure and shock (shock)
These side effects are rare (may affect up to 1 in 1,000 people)
Other side effects may include:
Very rare (may affect up to 1 in 10,000 people)
- Irritation at the application site.
Overdose symptoms may occur due to accelerated absorption (from the application site to the blood) or overdose (see also section 3, “If you have been given / if you use more Instillido than you should”).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the national reporting system Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Instillido
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label or carton after “EXP”. The expiry date is the last day of the month shown.
This medicine does not require special storage conditions. Store the blisters in the outer packaging to protect them from light.
Keep the pre-filled syringe in its blister pack until use.
Instillido syringes are intended for single use. The syringe and any unused gel should be discarded.
Medicines should not be disposed of via wastewater or household waste. Return any unused medicine to the pharmacy. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Instillido Composition
- The active ingredient is lidocaine, present as lidocaine hydrochloride monohydrate.
1 ml of gel contains 20.1 mg of lidocaine hydrochloride equivalent to 21.5 mg of lidocaine hydrochloride monohydrate.
6 ml - Pre-filled Syringe
A pre-filled syringe with 6 ml of gel contains 120.6 mg of lidocaine hydrochloride.
11 ml - Pre-filled Syringe
A pre-filled syringe with 11 ml of gel contains 221.1 mg of lidocaine hydrochloride.
- The other components (excipients) are: hypromellose, sodium hydroxide (for pH adjustment) and purified water.
Product Appearance and Container Content
Transparent, almost colorless, sterile gel.
Instillido is available in a sterile pre-filled syringe containing 6 ml or 11 ml of gel. The syringes are individually packaged in a sterile transparent blister.
Each graduation on the syringe is equivalent to approximately 1 ml of gel (20.1 mg of lidocaine hydrochloride).
Package Sizes:
Box of 10 pre-filled syringes with 6 ml of gel each. Box of 10 pre-filled syringes with 11 ml of gel each.
Only some package sizes may be marketed.
Marketing Authorization Holder
Farco-Pharma GmbH Gereonsmühlengasse 1-11
50670 Cologne Germany
Manufacturer
Klosterfrau Berlin GmbH Motzener Strasse 41
12277 Berlin,
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Member State | Medicinal Product Name |
Denmark | Instillido |
France | Glydo 20 mg/mL |
Germany | Instillido 20 mg/ml Gel in einer Fertigspritze |
Sweden | Instillido 20 mg/ml gel |
Netherlands | Instillido 20 mg/ml gel |
Belgium | Instillido 20 mg/ml gel |
Bulgaria | Instillido 20 mg/ml gel |
Croatia | Instillido 20 mg/ml gel |
Estonia | Instillido 20 mg/ ml geel |
Finland | Instillido 20 mg/ml geeli |
Lithuania | Lidocaine hydrochloride Instillido 20 mg/ml gelis |
Norway | Instillido 20 mg/ml gel i ferdigfylt sprøyte |
Poland | Instillido, 20 mg/mL, zel w ampulko-strzykawce |
Romania | Instillido 20 mg/ml gel |
Slovenia | Instillido 20 mg/ml gel v napolnjeni injekcijski brizgi |
Spain | Instillido 20 mg/ml gel |
United Kingdom (Northern Ireland) | Glydo 20 mg/ml gel in pre-filled syringe |
Date of the last revision of this prospectus:04/2023
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INSTILLIDO 20 mg/ml GELDosage form: DRESSING, 700 mgActive substance: lidocaineManufacturer: Grünenthal Pharma S.A.Prescription requiredDosage form: GEL/PASTE/ORAL LIQUID, 20 mg/gActive substance: lidocaineManufacturer: Chemische Fabrik Kreussler & Co GmbhPrescription not requiredDosage form: CREAM, 40 mg/gActive substance: lidocaineManufacturer: Isdin S.A.Prescription required
Online doctors for INSTILLIDO 20 mg/ml GEL
Discuss questions about INSTILLIDO 20 mg/ml GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















