INBRIJA 33 mg HARD CAPSULES FOR INHALATION POWDER

How to use INBRIJA 33 mg HARD CAPSULES FOR INHALATION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Inbrija 33 mg Powder for Inhalation, Hard Capsules
levodopa
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Inbrija and what is it used for
- What you need to know before you start using Inbrija
- How to use Inbrija
- Possible side effects
- Storing Inbrija
- Package Contents and Further Information
1. What is Inbrija and what is it used for
The active substance in Inbrija is levodopa. Inbrija is an inhaled medicine used to treat worsening of your symptoms during OFF periods of Parkinson's disease.
Parkinson's disease affects movement and is treated with a medicine you take regularly. During OFF periods, your usual medicine does not control the disease well enough and movement is likely to be more difficult.
You should continue taking your main Parkinson's disease medicine and use Inbrija to control worsening of symptoms (such as inability to move) during OFF periods.
2. What you need to know before you start using Inbrija
Do not use Inbrija
- if you are allergic to levodopaor to any of the other ingredients of this medicine (listed in section 6).
- if you have blurred vision, red eyes, severe eye pain and headache, halos around lights, your pupils are larger than usual and you feel dizzy. If you have any of these symptoms, you may have narrow-angle glaucoma, a condition that appears suddenly: stoptaking Inbrija and seek urgent medical attention.
- if you have a rare tumor of the adrenal glandcalled pheochromocytoma.
- if you are taking certain antidepressants called non-selective MAO inhibitors(e.g. isocarboxazid and phenelzine). You must stop taking these medicines at least 14 days before starting treatment with Inbrija. See also "Other medicines and Inbrija".
- if you have previously suffered from neuroleptic malignant syndrome, a potentially life-threatening reaction to certain medicines used to treat severe mental disorders, or if you have had non-traumatic rhabdomyolysis, a rare muscle disorder in which muscles are rapidly destroyed.
Warnings and precautions
Seek immediate medical attention if you havetremors, agitation, confusion, fever, rapid pulse or dizziness or fainting when standing up, or if you notice that your muscles become very stiff or you have violent muscle spasms. These may be symptoms of "medication withdrawal hyperpyrexia". For more information, see section 4.
Consult your doctor or pharmacistbefore starting to use Inbrija if you have, have had or develop:
- asthma, breathing difficulties such as chronic obstructive pulmonary disease (COPD) or other lung diseases or prolonged respiratory problems;
- any form of severe mental disorder, such as psychosis;
- heart attack or heartbeat-related problems. Your doctor will closely monitor you during the start of treatment;
- stomach or intestinal ulcers;
- an eye disorder called glaucoma, because in this case eye pressure should be monitored;
- severe kidney problems;
- severe liver problems.
If you are not sure if you have any of the symptoms mentioned, consult your doctor or pharmacist before using Inbrija.
Consult your doctor or pharmacistif you develop any of the following symptoms while using Inbrija:
- sudden attacks of sleepor you often feel very sleepy;
- changes or worsening of your mental conditionthat can be severe, such as psychotic behavior and suicidal thoughts;
- hallucinations, as well as confusion, difficulty sleeping, and vivid dreams. Alteration of mental activity, such as anxiety, depression, agitation, paranoia, delusional ideas or disorientation, aggressive behavior and delirium;
- worsening of any respiratory symptomsor if you have a respiratory infection;
- impulses or cravingsto behave in unusual ways or that you cannot resist the impulse, desire or temptation to perform certain activities that could be harmful to you or others. These behaviors are called impulse control disorders and may include addiction to gambling, eating or spending excessively and an abnormally high sexual desire or increased sexual thoughts or feelings. Your doctor may need to review your treatments.
- appearance or intensification of abnormal body movements(dyskinesia);
- dizziness when standing up(low blood pressure);
- melanoma(a type of skin cancer) or suspicious skin growths or marks.
If you are going to have surgery, inform your doctor that you are using Inbrija.
Tests
During prolonged treatment with your medicines, you may need to have heart, liver, kidney and blood tests. If you are going to have blood or urine tests, inform your doctor or nurse that you are taking Inbrija. This is because the medicine may affect the results of some tests.
Children and Adolescents
The use of Inbrija is not recommended in patients under 18 years of age.
Other medicines and Inbrija
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This is because some medicines may affect how Inbrija works.
Do not useInbrija if you have taken non-selective MAO inhibitor medicines for depression in the last 14 days. These medicines include isocarboxazid and phenelzine. If you are taking them, talk to your doctor or pharmacist before starting treatment with Inbrija.
Tell your doctor or pharmacistif you are taking:
- medicines for your Parkinson's disease called selective MAO inhibitors, such as rasagiline, selegiline and safinamide, COMT inhibitors like entacapone, tolcapone and opicapone, or anticholinergics like orphenadrine and trihexyphenidyl;
- medicines for mental illnesses including schizophrenia, such as benperidol, haloperidol, risperidone, chlorpromazine, fluphenazine decanoate, phenothiazine, butyrophenone or trifluoperazine;
- metoclopramide for nausea;
- isoniazid, an antibiotic for tuberculosis;
- medicines for high blood pressure, as the dose may need to be adjusted;
- medicines for depression called tricyclic antidepressants, such as clomipramine, desipramine or doxepin;
- amantadine for flu or Parkinson's disease.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Treatment with Inbrija is not recommended during pregnancy or in women of childbearing potential who are not using contraception.
Women should not breastfeed during treatment with Inbrija.
Driving and Using Machines
Inbrija may cause excessive drowsiness, dizzinessand sudden attacks of sleep. If you experience these symptoms, do not driveor use tools or machines. Before driving or using machines again, make sure you are no longer sleepy, dizzy or experiencing sudden attacks of sleep. Otherwise, you may put yourself or others at risk of serious injury or death.
3. How to Use Inbrija
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Before starting to take Inbrija, you must be regularly taking a treatment for Parkinson's disease that combines a dopa-decarboxylase inhibitor with levodopa.
The recommended dose of Inbrija is 2 capsulesfor the treatment of an OFF period. Do not use more than 2 capsules for each OFF period. You can use 2 capsules up to five times a day.
The maximum dose of Inbrija is 10 capsules per day.
Important information before using Inbrija:
- Inbrija hard capsules must not be swallowed.
- This medicine is for inhalation use only.
- Remove the capsules from the blister pack just before use.
- Two capsules of the medicine must be inhaled to receive the full dose.
- The medicine must only be used with the Inbrija inhaler.
- When opening a new box, always use the new inhaler.
- Your doctor or pharmacist will teach you how to use the medicine correctly.
See the "Instructions for Use" at the end of this package leaflet for how to use your medicine with the included inhaler.
If you use more Inbrija than you should
If you use more Inbrija than you should (or someone takes Inbrija accidentally), seek immediate medical attention.You may feel confused or agitated, and your heart rate may be slower or faster than usual.
If you forget to use Inbrija
Use Inbrija only during an OFF period. If the OFF period has passed, do not use Inbrija until the next OFF period occurs.
If you stop using Inbrija
Do not stop using Inbrija without checking with your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek immediate medical attention if you havean allergic edema with symptoms such as hives (urticaria), itching, rash, swelling of your face, lips, tongue or throat. This can cause difficulty breathing or swallowing.
Seek urgent medical attention ifyour muscles become very stiff or you have violent muscle spasms, tremors, agitation, confusion, fever, rapid pulse or severe fluctuations in your blood pressure. These may be symptoms of neuroleptic malignant syndrome (NMS, a rare severe reaction to medicines used to treat central nervous system disorders) or rhabdomyolysis (a rare severe muscle disease).
Seek urgent medical attention if you havestomach or intestinal bleeding, which can be detected as blood in your stools or black stools.
The use of this medicine may cause the following side effects:
Very Common(may affect more than 1 in 10 people):
- cough
Common(may affect up to 1 in 10 people):
- appearance or intensification of abnormal body movements (dyskinesia);
- infections of the nose, sinuses, throat or lungs;
- change in the color of mucus;
- change in the color of nasal mucus (i.e., not transparent);
- irritation or itching of the throat;
- feeling unwell (nausea); vomiting;
- tendency to fall.
Other side effects of unknown frequency that you may experience are:
- feeling of suffocation associated with the impact of the medicine powder on the back of the throat, immediately after use;
- skin cancer;
- red blood cell deficiency, which causes paleness and feeling of tiredness; increased tendency to contract infections due to a white blood cell deficiency; lack of platelets that can cause bruising and tendency to bleed;
- loss of appetite;
- confusion; hallucinations; depression; anxiety; nightmares; insomnia; alteration of mental activity and perceptions, loss of contact with reality; feeling agitated; suicidal behavior; disorientation; exaggerated feeling of happiness; increased libido; bruxism; paranoid or delusional feelings;
- movement disorder where the person's muscles contract uncontrollably; sudden, sometimes unpredictable, changes in symptoms due to the reappearance of Parkinson's disease symptoms; drowsiness; dizziness; worsening of Parkinson's disease; tingling; headache; tremors; convulsion; sudden onset of sleep; restless legs syndrome; ataxia (a disorder affecting coordination, balance, and speech); altered sense of taste; mental disorders affecting learning, memory, perception, and problem-solving; Horner's syndrome (an eye disease); dementia;
- blurred vision; double vision; enlarged pupils; eyes rolling back for a prolonged time; strong, involuntary closure of the eyelids;
- heart problems, a markedly rapid, strong or irregular heartbeat;
- low blood pressure shortly after standing up; high blood pressure; fainting; blood clot in a vein; hot flashes;
- shortness of breath; difficulty breathing; difficulty speaking; hiccups;
- stomach pain; constipation; diarrhea; dry mouth; stomach or intestinal bleeding; stomach ulcers; difficulty swallowing; indigestion; burning sensation in the mouth; flatulence; change in the color of saliva; excessive saliva;
- inflammation of the face, lips, tongue, limbs, and genitals; excessive sweating; rash; intense itching of the skin; a disease called Schoenlein-Henoch purpura, whose symptoms include a skin rash with purple spots; allergic reaction causing a rash of round, red, itchy patches on the skin; hair loss; change in the color of sweat;
- muscle spasms; trismus;
- difficulty emptying the bladder; change in the color of urine; loss of bladder control;
- painful and abnormally prolonged erection;
- swelling of the lower legs or hands; feeling of weakness and lack of energy; feeling tired; loss of energy; difficulty walking; chest pain;
- abnormal blood test results; weight loss; weight gain.
You may also experience the following side effects:
- inability to resist the impulse to perform an action that could be harmful, such as:
- strong impulse to gamble excessivelydespite serious personal or family consequences;
- altered or increased sexual desire and behavior that may be of concern to you or others, for example, an increased sexual impulse;
- excessive and uncontrolled shopping or spending;
- eating large amounts of food in a short period(binge eating) or compulsive eating(eating more than usual and more than needed to satisfy hunger).
Tell your doctor if you experience any of these behaviors; they will discuss with you ways to treat or reduce the symptoms.
Reporting Side Effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Inbrija
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister packs and carton after EXP or CAD. The expiry date is the last day of the month shown.
Store below 25°C. Store in the original package to protect from light and moisture and take out just before use.
Do not use the capsules if they are crushed, damaged or damp.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Inbrija Composition
- The active ingredient is levodopa. Each hard capsule contains 42 mg of levodopa. The dose that comes out of the inhaler mouthpiece (delivered dose) contains 33 mg of levodopa.
- The other components of the powder and capsule are colfosceril palmitate (DPPC), sodium chloride, hypromellose, titanium dioxide (E 171), carrageenan, potassium chloride, carnauba wax, corn starch, shellac, iron oxide black (E 172), propylene glycol, and potassium hydroxide.
Product Appearance and Container Contents
Inbrija inhalation powder, hard capsules, consists of a white powder for inhalation enclosed in hard, opaque capsules with "A42" printed in black on the capsule cap and two black stripes printed on the capsule body.
In this box, you will find an inhaler along with peelable blisters of 4 hard capsules each.
The pack sizes are
- a box with 16 hard capsules (strip of 4 blisters) and an inhaler
- a box with 32 hard capsules (strip of 8 blisters) and an inhaler
- a box with 60 hard capsules (strip of 15 blisters) and an inhaler
- a box with 92 hard capsules (strip of 23 blisters) and an inhaler
Only some pack sizes may be marketed.
Marketing Authorization Holder
Acorda Therapeutics Ireland Limited
10 Earlsfort Terrace
Dublin 2, D02 T380
Ireland
Tel: +353 (0)1 231 4609
Manufacturer
ADOH B.V.
Godfried Bomansstraat 31
6543 JA Nijmegen
Netherlands
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Lithuania Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Greece Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Luxembourg/Luxemburg Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Czech Republic Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Hungary Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Denmark Merz Therapeutics Nordics AB Gustav III S Boulevard 32 Regus Solna 169 73 Sweden Tel: +46 8 368000 | Malta Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Germany Merz Therapeutics GmbH Eckenheimer Landstraße 100 60318 Frankfurt/Main Tel: +49 (0) 69 1503 0 | Netherlands Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Estonia Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Norway Merz Therapeutics Nordics AB Gustav III S Boulevard 32 Regus Solna 169 73 Sweden Tel: +46 8 368000 |
Greece Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Austria Merz Pharma Austria GmbH Guglgasse 17 1110 Vienna Tel: +43 (0) 1 865 88 95 |
Spain Esteve Pharmaceuticals S.A. Passeig de la Zona Franca, 109, 4th floor 08038 Barcelona Spain Tel: +34 93 446 60 00 | Poland Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
France Merz Pharma France Tour EQHO 2, Avenue Gambetta 92400 Courbevoie Tel: +33 1 47 29 16 77 | Portugal Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Croatia Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 Ireland Merz Pharma UK Ltd. Suite B, Breakspear Park, Breakspear Way Hemel Hempstead Hertfordshire HP2 4TZ United Kingdom Tel: +44 (0) 208 236 0000 | Romania Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 Slovenia Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Iceland Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Slovakia Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Italy Merz Pharma Italia Srl Via Fabio Filzi 25 A 20124 Milan Tel: +39 02 66 989 111 | Finland Merz Therapeutics Nordics AB Gustav III S Boulevard 32 Regus Solna 169 73 Sweden Tel: +46 8 368000 |
Cyprus Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Sweden Merz Therapeutics Nordics AB Gustav III S Boulevard 32 Regus Solna 169 73 Tel: +46 8 368000 |
Latvia Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Date of the Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
----------------------------------------------------------------------------------------------------------------
Instructions for Use:
Read these instructions before starting to use Inbrija. | ||
Summary
| ||
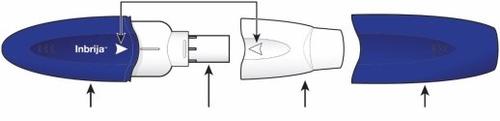
Components of your Inbrija inhaler Alignment arrows
Blue handle Capsule chamber White mouthpiece Blue cap | ||
Capsules | ||
Each box contains blisters of 4 capsules each.
| Prepare and use 2 capsules in total. Use one capsule at a time.
| Complete dose = 2 capsules.
|
Prepare your dose | ||
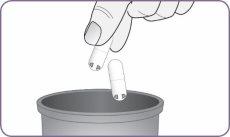
| Find a clean and dry surface. Make sure you have clean and dry hands. Take the inhaler and the strip of capsules. Open the package of 2 capsules. A complete dose is 2 capsules. | |
Step 2: Remove the blue cap from the inhaler
| Pull the cap to remove it. Set the cap aside. You will need it later to store the inhaler. | |
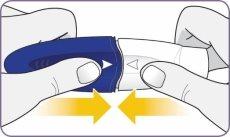
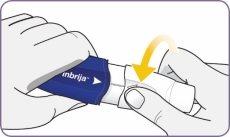
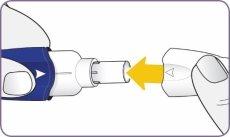
| Twist the mouthpiece and pull it to remove it from the handle. Set the mouthpiece and inhaler on a clean and dry surface. | |
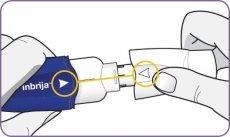
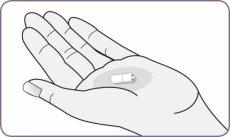
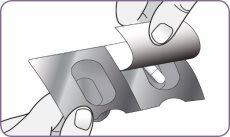
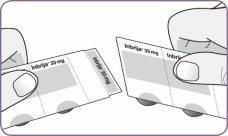
| Carefully peel the foil from the package and remove 1 capsule. Remove only 1 capsule at a time and just before using it. Do not use the capsules if they are crushed, damaged, or wet. In this case, discard it and take a new one. | |
| Hold the inhaler upright by the handle. Drop the first capsule into the capsule chamber. Do not load 2 capsules at the same time. |
Step 6: Assemble the white mouthpiece | |
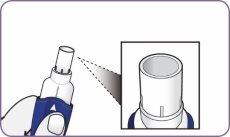
Align the arrows on the mouthpiece and handle
| Align the white arrows on the handle and mouthpiece. |
Press the mouthpiece only once
| Press the mouthpiece firmly against the handle until you hear a click. This action punctures the capsule. Do not press the handle and mouthpiece more than once. |
Release the mouthpiece
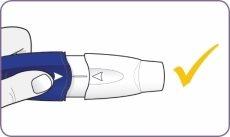
| Release the mouthpiece. The mouthpiece returns to its position and remains assembled. |
Now, the inhaler is ready to use. Do not press the handle and mouthpiece more than once. Otherwise, you could damage the capsule and not take the complete dose. If this happens, start again from step 4 using a new capsule. Check that the mouthpiece is firmly attached and does not fall off before proceeding to step 7. | |
Take your dose | |
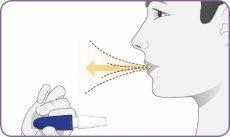
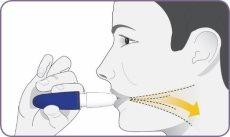
Step 7: Hold the inhaler away and exhale
| Stand or sit upright with your head and chest straight. Hold the inhaler at mouth level but away from it. Exhale completely. Do not exhale into the mouthpiece. |
Step 8: Inhale deeply to inhale the powder
| Hold the inhaler at mouth level, and close your lips firmly around the mouthpiece. Inhale deeply and widely until you feel your lungs are full. It is normal that it takes a few seconds. As you inhale, you will hear and feel the capsule "spin". This spin indicates that the inhaler is working and that you are receiving your medication. If you cough or interrupt the dose, start again from step 7 using the same capsule. Important: if you have not heard or felt the capsule spin during inhalation, you may need to inhale more deeply and longer, or you may need to clean the mouthpiece. (Do not rinse the mouthpiece or wet the inhaler). Refer to step 13: Cleaning the mouthpiece. Start again from step 7 using the same capsule. |
Step 9: Hold your breath for 5 seconds and exhale
| Remove the inhaler from your mouth and hold your breath for 5 seconds. Then, exhale. |
Step 10: Remove the capsule from the inhaler | |
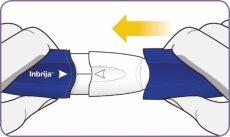
Twist and remove the mouthpiece
| Twist and remove the mouthpiece. |
Remove the used capsule
| Remove the used capsule. |
Step 11: Dose with the 2nd capsule
| Repeat steps 4 to 10 with a second capsule to complete the full dose. You must inhale the contents of the second capsule within 10 minutes of the first. |
Disposal and Storage | |
Step 12: Disposal of used capsules
| Dispose of used capsules according to local regulations. |
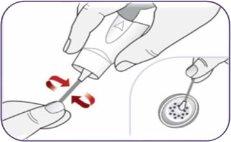
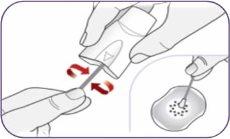
Step 13: Cleaning the mouthpiece It is normal for some powder to remain on or in the inhaler. To avoid accumulation, remove the powder from the mouthpiece holes when necessary by making circular movements with a new dry cotton swab. | |
Cleaning the upper holes of the mouthpiece
| Clean the upper holes of the mouthpiece. |
Cleaning the lower holes of the mouthpiece
| Clean the lower holes of the mouthpiece. |
You can also use a dry paper towel to wipe the outside of the mouthpiece when necessary. Do not clean any other part of the inhaler. Do not rinse the mouthpiece or wet the inhaler. | |
Step 14: Store the inhaler | |
Check that there is no capsule in the inhaler
| Check that there is no capsule in the inhaler before storing it. |
Assemble the mouthpiece
| Assemble the mouthpiece onto the handle by pressing it until you hear a click. |
Replace the cap
| Replace the cap over the mouthpiece. |
Ready to store
| Now the inhaler is ready to store. |
Inhaler Cleaning
|
- Country of registration
- Average pharmacy price354.55 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INBRIJA 33 mg HARD CAPSULES FOR INHALATION POWDERDosage form: TABLET, 10 mg/100 mgActive substance: levodopa and decarboxylase inhibitorManufacturer: Fairmed Healthcare GmbhPrescription requiredDosage form: TABLET, 12.5 mg/50 mgActive substance: levodopa and decarboxylase inhibitorManufacturer: Fairmed Healthcare GmbhPrescription requiredDosage form: TABLET, 25 mg/100 mgActive substance: levodopa and decarboxylase inhibitorManufacturer: Fairmed Healthcare GmbhPrescription required
Online doctors for INBRIJA 33 mg HARD CAPSULES FOR INHALATION POWDER
Discuss questions about INBRIJA 33 mg HARD CAPSULES FOR INHALATION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







 Step 1: Preparation
Step 1: Preparation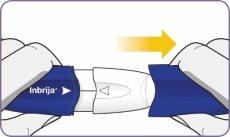
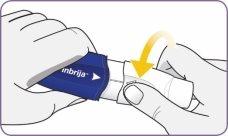 Step 3: Twist and remove the white mouthpiece
Step 3: Twist and remove the white mouthpiece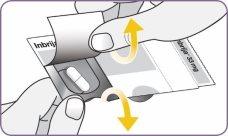 Step 4: Remove 1 capsule from the package
Step 4: Remove 1 capsule from the package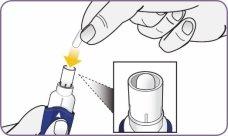 Step 5: Load the capsule
Step 5: Load the capsule