
INAQOVI 35 MG/100 MG FILM-COATED TABLETS

How to use INAQOVI 35 MG/100 MG FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
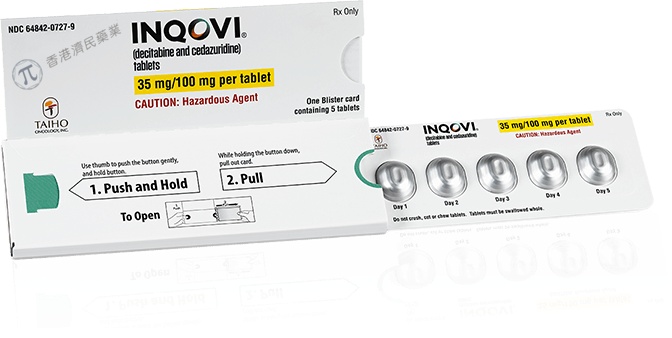
Inaqovi 35 mg/100 mg film-coated tablets
decitabine/cedazuridine
(decitabine/cedazuridine)
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Inaqovi and what is it used for
- What you need to know before you take Inaqovi
- How to take Inaqovi
- Possible side effects
- Storage of Inaqovi
- Contents of the pack and other information
1. What is Inaqovi and what is it used for
What is Inaqovi
Inaqovi is a cancer medicine. It contains the active substances decitabine and cedazuridine.
What is Inaqovi used for
Inaqovi is used alone to treat acute myeloid leukaemia (AML) in adults when chemotherapy is not considered suitable. You will be given Inaqovi when you are first diagnosed with AML.
AML is a type of cancer that affects the white blood cells in the blood called myeloid cells. In AML, the myeloid cells multiply and grow very quickly in the bone marrow and blood.
How Inaqovi works
Inaqovi contains two active substances that work in different ways. Decitabine works by stopping the growth of cancer cells. It also kills cancer cells. Cedazuridine does not directly affect cancer cells, but it prevents the breakdown of decitabine. This increases the amount of decitabine available in the body and helps to increase the effects of decitabine.
2. What you need to know before you take Inaqovi
Do not take Inaqovi
- if you are allergic to decitabine or cedazuridine or any of the other ingredients of this medicine (listed in section 6);
- if you are breast-feeding (see section 2 Breast-feeding).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start taking Inaqovi if:
- you have lung problems;
- you have liver problems;
- you have kidney problems;
- you have heart problems.
Myluosuppression and differentiation syndrome
Inaqovi may cause severe myelosuppression (a condition in which the bone marrow cannot produce enough blood cells) or a severe immune reaction called 'differentiation syndrome'. Both can be life-threatening.
Seek urgent medical attention if you notice any signs or symptoms (see section 4).
Cardiovascular disease
Talk to your doctor if you have a history of heart problems so that they can monitor you for signs and symptoms of heart failure.
Blood tests
You will have blood tests during treatment. These will be done before you start treatment with Inaqovi, at the start of each treatment cycle, or if you notice any signs or symptoms of myelosuppression. These tests are to check that:
- you have enough blood cells, and
- your liver and kidneys are working properly.
Your doctor may change or delay your dose of Inaqovi. Your doctor may also give you medicines to help prevent infections.
Children and adolescents
Inaqovi must not be given to children and adolescents under 18 years. This medicine has not been studied in this age group.
Other medicines and Inaqovi
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines before you start treatment with Inaqovi. Inaqovi may affect how some medicines work, especially if you are also taking medicines to treat:
- cancer, such as cytarabine, gemcitabine, or azacitidine.
Pregnancy, contraception, breast-feeding, and fertility
Pregnancy
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Do not take Inaqovi during pregnancy, as it may harm the unborn baby. If you can become pregnant, a pregnancy test is recommended before starting treatment with Inaqovi.
Contraception
Women who can become pregnant must use effective contraception during treatment with Inaqovi and for 6 months after the last dose of Inaqovi.
Men with partners who can become pregnant must use effective contraception during treatment with Inaqovi and for 3 months after the last dose of Inaqovi.
Talk to your doctor about the most effective methods of contraception.
Breast-feeding
Do not breast-feed during treatment with Inaqovi. This is because it is not known whether Inaqovi passes into breast milk and whether this could harm your baby.
Male and female fertility
Inaqovi may affect fertility. It is not known whether the effect on fertility is permanent. Talk to your doctor before taking this medicine if you have any questions or if you want to preserve your sperm or freeze your eggs before starting treatment.
Driving and using machines
Inaqovi may affect your ability to drive or use tools or machines. If you feel tired or dizzy after taking Inaqovi, do not drive or use tools or machinery until you feel better.
Inaqovi contains lactose and sodium
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
This medicine contains less than 1 mmol sodium (23 mg) per tablet, which is essentially 'sodium-free'.
3. How to take Inaqovi
This medicine will be prescribed by a doctor with experience in the use of cancer medicines. Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor again.
The recommended dose is 1 tablet once a day for the first 5 days of a treatment cycle. This is followed by 23 days without taking this medicine. A treatment cycle is 28 days.
- Swallow the tablets whole, with water, at about the same time each day.
- Do not chew, crush, or break the tablets to avoid skin contact or spilling the powder into the air.
- Since taking Inaqovi with food may decrease the effectiveness of the medicine, Inaqovi should be taken without food. Take Inaqovi 2 hours before or 2 hours after a meal.
You will usually take Inaqovi for at least 4 cycles. Your doctor will do regular blood tests to check your response to treatment. Your doctor may delay the dose and change the total number of cycles, depending on how you respond to treatment.
If you vomit
If you vomit after taking a dose, do not take another dose that day. Take the next dose at the usual time the next day.
Your doctor may prescribe an additional medicine for you to take before each dose of Inaqovi to prevent nausea or vomiting during treatment.
If you take more Inaqovi than you should
An overdose may cause myelosuppression, sepsis, or pneumonia (see section 4 Possible side effects). If you take more Inaqovi than you should, seek urgent medical attention.
If you forget to take Inaqovi
If you miss a dose and it has been less than 12 hours since the time you usually take it, you should take the missed dose as soon as possible and continue with your normal daily dose schedule.
If you miss a dose and it has been 12 hours or more, do not take the missed dose. Take the next dose the next day at the usual time. Extend the dosing period by one day for each missed dose. Make sure to complete a total of 5 daily doses for each cycle.
If you stop taking Inaqovi
If you stop taking this medicine, it is possible that your cancer will no longer be controlled and the symptoms of your cancer may come back. Therefore, you should only stop taking this medicine if your doctor tells you to.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor, pharmacist, or nurse immediately if you notice any of the following serious side effects:
- Fever: may be a sign of an infection caused by low white blood cell counts (very common: may affect more than 1 in 10 people).
- Chest pain or difficulty breathing (with or without fever or cough): may be signs of pneumonia (very common: may affect more than 1 in 10 people) or inflamed lungs (interstitial lung disease: frequency not known).
- Bleeding, including blood in your stools or bleeding nose or easy bruising: may be a sign of low blood cell counts (platelets and red blood cells) (common: may affect up to 1 in 10 people).
- Difficulty moving, speaking, or understanding, or severe headache, seizures, numbness, or weakness in any part of your body: may be signs of bleeding in the brain (common: may affect up to 1 in 10 people).
- Feeling dizzy or faint, confusion or disorientation, weakness, shortness of breath, decreased urination, diarrhea, nausea/vomiting, fever, chills, or feeling very cold, sweating, or cough: may be signs and symptoms of a blood infection (sepsis) (very common: may affect more than 1 in 10 people).
- Fever, cough, difficulty breathing, skin rash, decreased urination, low blood pressure, swelling of arms or legs, and rapid weight gain: may be signs of a severe immune reaction (differentiation syndrome) (frequency not known).
Other side effects:
Very common(may affect more than 1 in 10 people)
- urinary tract infection;
- bacterial, viral, or fungal infection;
- high blood sugar levels;
- mouth or tongue ulcers due to painful inflammation of the mouth lining;
- diarrhea;
- nausea and vomiting;
- abnormal liver function tests (increased ALAT, ASAT, alkaline phosphatase, bilirubin).
Common(may affect up to 1 in 10 people)
- sinusitis;
- headache;
- inflamed intestine (neutropenic colitis).
Uncommon(may affect up to 1 in 100 people)
- decrease in the number of red blood cells, white blood cells, and platelets;
- sudden fever with multiple painful red or reddish-purple raised spots on the skin, usually on the arms, legs, trunk, face, or neck ('Acute febrile neutrophilic dermatosis' or Sweet's syndrome');
- heart muscle disease.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Inaqovi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after 'EXP' and on the blister strip after 'EXP'. The expiry date refers to the last day of the month shown.
Store in the original package to protect from moisture.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Inaqovi contains
- The active substances are decitabine and cedazuridine. Each film-coated tablet contains 35 mg of decitabine and 100 mg of cedazuridine.
- The other ingredients are:
Inaqovi contains lactose and sodium, see section 2
Tablet core
Lactose monohydrate, hypromellose (E464), croscarmellose sodium (E466), colloidal anhydrous silica, magnesium stearate (E572).
Film coating
Polyvinyl alcohol (E1203), titanium dioxide (E171), polyethylene glycol (E1521), talc (E553b), red iron oxide (E172).
Appearance and packaging
Inaqovi are red, oval, biconvex, film-coated tablets, 14 mm in diameter, smooth on one side and with 'H35' engraved on the other side.
They are supplied in aluminium blisters containing 5 tablets.
Marketing authorisation holder
Otsuka Pharmaceutical Netherlands B.V.
Herikerbergweg 292
1101 CT Amsterdam
Netherlands
Manufacturer
BSP Pharmaceuticals S.p.A.
Via Appia Km. 65,561
04013 Latina Scalo (LT)
Italy
R-PHARM Germany GmbH
Heinrich-Mack-Straße 35
89257 Illertissen
Germany
You can ask for more information about this medicine from the marketing authorisation holder.
België/Belgique/Belgien Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | Lietuva Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | Luxembourg/Luxemburg Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Ceská republika Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | Magyarország Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Danmark Otsuka Pharma Scandinavia AB Tlf: +46 (0) 8 545 286 60 | Malta Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Deutschland Otsuka Pharma GmbH Tel: +49 (0) 69 1700 860 | Nederland Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Eesti Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | Norge Otsuka Pharma Scandinavia AB Tlf: +46 (0) 8 545 286 60 |
Ελλάδα Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | Österreich Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
España Otsuka Pharmaceutical, S.A. Tel: +34 93 208 10 20 | Polska Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
France Otsuka Pharmaceutical France SAS Tél: +33 (0)1 47 08 00 00 | Portugal Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Hrvatska Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | România Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Ireland Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | Slovenija Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Ísland Vistor hf. Sími: +354 (0) 535 7000 | Slovenská republika Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Italia Otsuka Pharmaceutical Italy S.r.l. Tel: +39 (0) 2 0063 2710 | Suomi/Finland Otsuka Pharma Scandinavia AB Tlf: +46 (0) 8 545 286 60 |
Κύπρος Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | Sverige Otsuka Pharma Scandinavia AB Tlf: +46 (0) 8 545 286 60 |
Latvija Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 | United Kingdom (Northern Ireland) Otsuka Pharmaceutical Netherlands B.V. Tel: +31 (0) 20 85 46 555 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site: https://www.ema.europa.eu. There are also links to other web sites about rare diseases and orphan medicines.
The leaflet for this medicine can be found in all EU/EEA languages on the European Medicines Agency web site.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INAQOVI 35 MG/100 MG FILM-COATED TABLETSDosage form: TOPICAL SOLUTION, 5 mg/g + 100 mg/gActive substance: fluorouracil, combinationsManufacturer: Almirall Hermal GmbhPrescription requiredDosage form: INJECTABLE, 25 mg/mlActive substance: azacitidineManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 25 mg/mlActive substance: azacitidineManufacturer: Betapharm Arzneimittel GmbhPrescription required
Online doctors for INAQOVI 35 MG/100 MG FILM-COATED TABLETS
Discuss questions about INAQOVI 35 MG/100 MG FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






