
IBEROGAST ORAL DROPS IN SOLUTION


How to use IBEROGAST ORAL DROPS IN SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT
Iberogast
Oral drops in solution
Liquid ethanol extracts of white hawthorn, angelica roots, chamomile flowers, caraway fruits, milk thistle fruits, lemon balm leaves, peppermint leaves, celandine, and licorice root.
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
Follow exactly the administration instructions of the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this package leaflet. See section 4.
- You should consult a doctor if your symptoms worsen or do not improve after 7 days of treatment.
Contents of the package leaflet
- What is Iberogast and what is it used for
- What you need to know before taking Iberogast
- How to take Iberogast
- Possible side effects
- Storage of Iberogast
- Package contents and additional information
1. What is Iberogast and what is it used for
Iberogast is a plant-based medication that contains liquid ethanol extracts of white hawthorn, angelica roots, chamomile flowers, caraway fruits, milk thistle fruits, lemon balm leaves, peppermint leaves, celandine, and licorice root.
This medication is indicated for the treatment of gastrointestinal disorders such as dyspepsia (digestive disorder) and gastritis (stomach inflammation), as well as for the relief of associated symptoms, stomach pain, abdominal bloating, flatulence, gastrointestinal colic, nausea, and heartburn.
Iberogast is indicated for adults and adolescents over 12 years of age.
You should consult a doctor if your symptoms worsen or do not improve after 7 days of treatment.
2. What you need to know before taking Iberogast
Do not take Iberogast
If you are allergic to the active substances or to any other component of this medication (listed in section 6).
If you have or have had liver disease or if you are taking medications that include liver damage as a side effect in the package leaflet. In case of doubt, consult your doctor or pharmacist.
Warnings and precautions
Consult your doctor, pharmacist, or nurse if symptoms persist or if the expected results are not obtained despite treatment.
Be aware of signs and symptoms that may indicate that your liver is not functioning properly.
If you notice that your skin or eyes are yellowish, your urine is dark, your stools are discolored, or you have pain in the upper abdomen, stop taking Iberogast immediately and consult your doctor. These may be symptoms of liver damage.
Children and Adolescents
Children under 12 years of age should not use this medication, as there is not enough clinical information available.
If your symptoms do not improve within 7 days or worsen, consult your doctor so that they can rule out other serious diseases.
Other medications and Iberogast
No interactions with other medications are known to date.
Inform your doctor or pharmacist if you are taking, have recently taken, or may take any other medication.
Taking Iberogast with food and drinks
No interactions with food and drinks are known.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
No studies have been conducted to establish the safety of using this medication during pregnancy or breastfeeding, so it should not be administered in these circumstances.
Driving and using machines
Iberogast contains ethanol (alcohol). At the recommended doses of the medication, the amounts of alcohol ingested are not expected to affect driving ability or machine operation. However, it may decrease reaction time, so it is not recommended to drive vehicles or operate machinery that requires special attention until it is verified that the ability to perform these tasks is not affected.
Important information about certain ingredients of Iberogast
Iberogast contains 31% (V/V) ethanol (alcohol), which corresponds to 240 mg of ethanol in 20 drops (per unit dose), equivalent to 6.2 ml of beer or 2.6 ml of wine. This medication is harmful to people with alcoholism. The alcohol content should be taken into account in the case of pregnant or breastfeeding women, children, and high-risk populations, such as patients with liver disease or epilepsy.
Iberogast contains less than 0.1 IU per 20 drops (unit dose).
3. How to take Iberogast
Follow exactly the administration instructions of this medication contained in this package leaflet or as indicated by your doctor, pharmacist, or nurse. In case of doubt, ask your doctor, pharmacist, or nurse.
The normal recommended dose is:
Adults and adolescents over 12 years of age: take 20 drops of Iberogast, 3 times a day before or during meals, with a little liquid. The bottle should be tilted during dripping (at a 45-degree angle).
If symptoms persist or worsen after 7 days of treatment, consult your doctor.
After 2 months of using the medication, consult your doctor about the possibility of continuing treatment. The duration of treatment depends on the type, severity, and evolution of the disease.
Remember to shake the medication before use.
Use in children and adolescents:
Administration of this medication is not recommended for children under 12 years of age.
If you take more Iberogast than you should
To date, there have been no cases of acute overdose. However, the alcohol content present in the product should be considered.
In case of overdose, the described side effects may be enhanced. In case of accidental ingestion, consult your pharmacist or doctor immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the product and the amount ingested.
If you forget to take Iberogast
If you have forgotten to take Iberogast, the next dose will be taken as described in the instructions of this package leaflet or according to the dose indicated by your doctor. Do not take a double dose to make up for the forgotten dose.
If you have any other questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, Iberogast can cause side effects, although not everyone will experience them.
Very rare(may affect up to 1 in 10,000 people): hypersensitivity reactions such as skin rashes, itching, or difficulty breathing.
Frequency not known(cannot be estimated from available data): liver damage (increased liver values, drug-related jaundice, hepatitis, and liver failure) has been reported; if you notice symptoms such as yellowish skin or eyes, dark urine, discolored stools, or pain in the upper abdomen, stop taking Iberogast immediately and consult your doctor. These may be symptoms of liver damage.
In case of a reaction to the medication, stop taking the medication and consult a doctor immediately. They will be able to assess the severity of the reaction and determine any additional necessary measures.
If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this package leaflet.
Reporting side effects:
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Iberogast
Keep this medication out of the sight and reach of children.
Do not store at a temperature above 25°C.
Do not use this medication after the expiration date shown on the packaging after EXP. The expiration date is the last day of the month indicated.
Consume Iberogast within 8 weeks after opening the packaging.
Medications should not be thrown down the drain or into the trash. Deposit the packaging and medications you no longer need at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Iberogast
Each ml of oral drops (equivalent to 20 drops) contains:
Liquid ethanol extract (50% (V/V)):
0.15 ml of fresh white hawthorn plants (Iberis amara L) (1:1.5-2.5);
Liquid ethanol extracts (30% (V/V)):
0.2 ml of chamomile flowers (Matricaria recutita L) (1:2-4);
0.1 ml of angelica root (Angelica archangelica L) (1:2.5-3.5);
0.1 ml of caraway fruit (Carum carvi L) (1:2.5-3.5);
0.1 ml of celandine (Chelidonium majus L) (1:2.5-3.5);
0.1 ml of licorice root (Glycyrrhiza glabra L) (1:2.5-3.5);
0.1 ml of lemon balm leaves (Melissa officinalis L) (1:2.5-3.5);
0.1 ml of milk thistle fruit (Silybum marianum L Gaertner) (1:2.5-3.5) and
0.05 ml of peppermint leaves (Mentha piperita L) (1:2.5-3.5)
The other components (excipients) are:
The medication contains 31% (V/V) ethanol.
Appearance of the product and package contents
Dark brown liquid, transparent or slightly turbid with a characteristic odor and bitter taste.
Due to the characteristics of the medication, it may happen that Iberogast presents precipitates or turbidity; if this occurs, it will not affect the efficacy of the preparation.
The medication is presented in a brown glass bottle with a dropper and screw cap. It is available in 3 package sizes: 20 ml, 50 ml, and 100 ml.
Marketing authorization holder
Bayer Hispania, S.L.
Av. Baix Llobregat, 3-5
08970 Sant Joan Despí (Barcelona)
Manufacturer:
Steigerwald Arzneimittelwerk GmbH
Havelstrasse 5
Darmstadt D-64295
Germany
Date of the last revision of this package leaflet: April 2019
"Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)http://www.aemps.es/"
INSTRUCTIONS FOR USING THE DROPPER
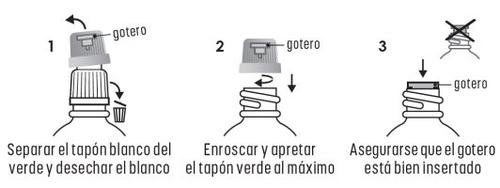
- Country of registration
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IBEROGAST ORAL DROPS IN SOLUTIONDosage form: CHEWABLE TABLET, 40 mg simethiconeActive substance: siliconesManufacturer: Uriach Consumer Healthcare S.L.Prescription not requiredDosage form: CHEWABLE TABLET, 120 mgActive substance: siliconesManufacturer: Uriach Consumer Healthcare S.L.Prescription not requiredDosage form: CAPSULE, 240 mgActive substance: siliconesManufacturer: Uriach Consumer Healthcare S.L.Prescription not required
Online doctors for IBEROGAST ORAL DROPS IN SOLUTION
Discuss questions about IBEROGAST ORAL DROPS IN SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















