
HYMPAVZI 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN

How to use HYMPAVZI 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Hympavzi 150 mg solution for injection in pre-filled pen
marstacimab
This medicinal product is subject to additional monitoring, which will allow quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Hympavzi and what is it used for
- What you need to know before you use Hympavzi
- How to use Hympavzi
- Possible side effects
- Storage of Hympavzi
- Contents of the pack and other information
1. What is Hympavzi and what is it used for
Hympavzi contains the active substance marstacimab. Marstacimab is a monoclonal antibody, a type of protein designed to recognize a specific target in the body, called tissue factor pathway inhibitor (TFPI), and bind to it.
Hympavzi is a medicine used to prevent or reduce bleeding in patients aged 12 years and older and weighing at least 35 kg with:
- severe haemophilia A (congenital factor VIII deficiency, when the factor VIII level in the blood is below 1%), who have not developed factor VIII inhibitors, or
- severe haemophilia B (congenital factor IX deficiency, when the factor IX level in the blood is below 1%) who have not developed factor IX inhibitors.
Haemophilia A is a bleeding disorder caused by the lack of factor VIII. Haemophilia B is a bleeding disorder caused by the lack of factor IX. Factor VIII and factor IX are essential proteins for blood to clot and stop bleeding. Some patients with haemophilia may develop inhibitors to factor VIII or factor IX (blood antibodies that act against factor VIII or factor IX replacement medicines and prevent them from working properly).
The active substance in Hympavzi, marstacimab, recognizes and binds to TFPI, a protein that prevents blood from clotting too much. By binding to TFPI, marstacimab reduces its function, which triggers the formation of thrombin (a protein that plays an essential role in blood clotting when an injury or wound occurs in the body). This helps to improve clotting and stop bleeding in patients with haemophilia.
2. What you need to know before you use Hympavzi
Do not use Hympavzi
- if you are allergic to marstacimab or any of the other ingredients of this medicine (listed in section 6). If you are not sure if you are allergic, talk to your doctor, pharmacist, or nurse before using Hympavzi.
Warnings and precautions
Talk to your doctor before you start using Hympavzi.
Before you start using Hympavzi, it is very important that you talk to your doctor about using other factor VIII and factor IX products(products that contribute to blood clotting in a way different from Hympavzi) at the same time as using Hympavzi. You may need to use other factor VIII or factor IX products to treat spontaneous bleeding episodes while using Hympavzi. Follow your healthcare professional's instructions exactly on when and how to use these factor VIII or factor IX products at the same time as using Hympavzi.
Blood clots (thromboembolic events)
Hympavzi increases the blood's ability to clot. It is known that medicines like Hympavzi can cause blood clots in blood vessels (called thromboembolic events). Blood clots can be life-threatening. Tell your doctor if you have a history of blood clots or if you have a disease that increases the risk of blood clots, such as:
- history of coronary artery disease (heart disease caused by narrowing or blockage of the blood vessels that supply blood to the heart muscle)
- history of ischaemic disease (reduced blood flow due to narrowing or blockage of blood vessels)
- history of blood clots in veins or arteries
- severe infections
- sepsis (blood infection)
- trauma or crush injuries
- cancer
Stop using Hympavzi and talk to a doctor immediatelyif you notice any symptoms of a possible blood clot, including the following side effects:
|
|
legs |
|
|
|
|
|
| |
|
Allergic reactions
Symptoms of an allergic reaction have been seen in people using Hympavzi. Stop using Hympavzi and seek medical attention immediately if you notice any symptoms of a severe allergic reaction, including:
|
|
throat |
|
Children under 12 years of age
Hympavzi should not be used in children under 1 year of age, and it is not recommended in children under 12 years of age. The safety and benefits of this medicine in this population are still unknown.
Other medicines and Hympavzi
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, contraception, and breastfeeding
If you can become pregnant, you must use an effective method of contraception during treatment with Hympavzi and for at least 1 month after the last injection of Hympavzi.
If you are pregnant, think you may be pregnant, or plan to become pregnant, talk to your doctor before using this medicine. Your doctor will consider the benefits of your treatment with Hympavzi and the risks to the fetus.
If you are breastfeeding, talk to your doctor if you should stop breastfeeding or stop using Hympavzi. Your doctor will consider the benefits of your treatment with Hympavzi and the benefits to your baby.
Driving and using machines
Hympavzi has a negligible or limited influence on the ability to drive or use machines.
Hympavzi contains polisorbate 80
This medicine contains 0.2 mg of polisorbate 80 per ml. Polysorbates may cause allergic reactions. Talk to your doctor if you have any known allergies.
Hympavzi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 1 ml; this is essentially "sodium-free".
3. How to use Hympavzi
You will start your treatment under the supervision of a qualified doctor experienced in treating patients with haemophilia. Follow your doctor's instructions for administering the medicine exactly. If you are unsure, ask your doctor or pharmacist.
Your healthcare professional will provide you with instructions on how to stop your current treatment when switching to Hympavzi. If you are unsure what to do, contact your healthcare professional.
If you have a severe illness or need to undergo major surgery, inform your healthcare professional that you are using Hympavzi.
Keep a record
Each time you use Hympavzi, write down the name and batch number of the medicine.
How Hympavzi is administered
- Hympavzi is administered via an injection under the skin (subcutaneous injection). Do not inject it into a vein or muscle.
- To make the injection less painful, Hympavzi can be left at room temperature between 15 and 30 minutes inside the box and away from direct sunlight. Do not heat Hympavzi in any other way, such as in hot water or in a microwave.
Before using the pen for the first time, your doctor, nurse, or pharmacist will show you and/or your caregiver how to inject Hympavzi. If you or your caregiver administer the Hympavzi injection, you must read and follow the detailed instructions for use on the back of this leaflet.
Where to inject Hympavzi
- Your doctor or nurse will show you and/or your caregiver where on the body to inject Hympavzi. The best place to administer Hympavzi is the abdomen or thigh. Injections into the buttocks should only be given by a caregiver, doctor, or nurse.
- Change the injection site each time you receive an injection.
- If more than one injection is needed to administer a full dose, each injection should be given in a different injection site.
- If you are using other medicines that need to be injected under the skin, they should be administered in different injection sites.
How much Hympavzi to use
Your treatment will start with a loading dose, after which you will receive a maintenance dose once a week.
- Loading dose (a higher initial dose to quickly increase levels in the body): The recommended dose is 300 mg.
- Maintenance dose: The recommended dose is 150 mg.
You should use this medicine once a week (at any time of day), on the same day each week.
You should keep a record of which day of the week you use Hympavzi to help you remember to inject this medicine once a week.
Depending on how you respond to Hympavzi, your doctor may change your maintenance dose as needed, up to a maximum of 300 mg per week.
Use in adolescents
Hympavzi can be used in adolescents aged 12 years and older. An adolescent may administer the medicine injection, provided that their doctor or nurse and parent or caregiver agree and the patient is prepared to do so.
If you use more Hympavzi than you should
If you have used more Hympavzi than you should, talk to your doctor immediately. You may be at risk of developing side effects such as blood clots and may need medical attention. Follow your doctor's instructions for administering Hympavzi exactly, and if you are unsure, ask your doctor, pharmacist, or nurse.
If you forget to use Hympavzi
- If you forget to administer the dose on the scheduled day, inject the missed dose as soon as possible before the next scheduled dose. Then, continue injecting the medicine as scheduled. Do not inject a double dose to make up for missed doses.
- If you forget two scheduled doses in a row (i.e., if more than 13 days have passed since your last injection), contact your doctor as soon as possible and ask what to do.
- If you are unsure what to do, contact your doctor, pharmacist, or nurse.
If you stop using Hympavzi
Do not stop using Hympavzi without talking to your doctor. If you stop using Hympavzi, you may lose protection against bleeding.
If you have any other questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Hympavzi may cause a rash (in up to 1 in 100 people), which can be severe. Talk to your doctor immediately if you have a severe rash, as some can be serious. Do not use Hympavzi again until you have talked to your doctor about the rash.
Stop using Hympavzi and talk to a doctor immediately if you notice any signs or symptoms of a possible blood clot. See the list of possible symptoms of a blood clot (thromboembolic event) in section 2, "What you need to know before you use Hympavzi".
Other side effects
Tell your doctor or nurse if you have any of the following side effects.
Very common(may affect more than 1 in 10 people)
- reaction at the injection site (including itching, swelling, redness, pain, bruising, and hardening)
Common(may affect up to 1 in 10 people)
- headache
- high blood pressure
- itching (pruritus)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hympavzi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pre-filled pen and on the carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the pre-filled pen in the original package to protect it from light.
Hympavzi can be taken out of the refrigerator and stored at room temperature (up to 30°C) in the original package for a maximum of 7 days. After storing Hympavzi at room temperature, do not put it back in the refrigerator. If Hympavzi has been at room temperature for more than 7 days, discard it, even if it contains unused medicine.
Do not shake.
Take Hympavzi out of the refrigerator before use. To make the injection less painful, Hympavzi can be left at room temperature between 15 and 30 minutes inside the box and away from direct sunlight.
Before using the medicine, check that the solution does not contain particles or has changed colour. Do not use the medicine if it is cloudy, has a dark yellow colour, or contains flakes or particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Hympavzi
- The active ingredient is marstacimab.
- The other components are disodium edetate, L-histidine, L-histidine monohydrochloride, polysorbate 80 (E 433), sucrose, and water for injectable preparations (see section 2, "Hympavzi contains polysorbate 80" and "Hympavzi contains sodium").
Appearance of the Product and Container Contents
Hympavzi is a clear and colorless to light yellow injectable solution (injection) in a pre-filled pen.
Each Hympavzi container contains 1 pre-filled pen.
Marketing Authorization Holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelles
Belgium
Manufacturer
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Belgium
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel: +370 5 251 4000 |
| Magyarország Pfizer Kft. Tel.: +36 1 488 37 00 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +356 21344610 |
Danmark Pfizer ApS Tlf.: +45 44 20 11 00 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
| Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
España Pfizer, S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L. Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: +386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Tel: +1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: +421 2 3355 5500 |
Ísland Icepharma hf. Sími: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
| United Kingdom (Northern Ireland) Pfizer Limited Tel: +44 (0) 1304 616161 |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel: +371 670 35 775 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: https://www.ema.europa.eu.
INSTRUCTIONS FOR USE
Hympavzi 150 mg solution for injection in pre-filled pen
These "Instructions for Use" contain information on how to inject Hympavzi.
Read these "Instructions for Use" carefully before using the Hympavzi pre-filled pen and each time you renew your prescription, as there may be new information.
Your doctor, nurse, or pharmacist should show you or your caregiver how to properly prepare and inject a dose of Hympavzi before you use it for the first time.
Do not inject Hympavzi or have it injected by anyone until you have been shown how to perform the injection.
Important Information
- Each Hympavzi pre-filled pen is a single-dose pre-filled pen (called a "pen" in these "Instructions for Use"). The Hympavzi pre-filled pen contains 150 mg of Hympavzi for subcutaneous injection (under the skin).
- Do notinject Hympavzi into a vein or muscle.
- Do notshake Hympavzi.
- To help you remember when to inject Hympavzi, you can mark your calendar in advance. Call your doctor, nurse, or pharmacist if you or your caregiver have any questions about the correct way to inject Hympavzi.
Storage of Hympavzi
- Store unused Hympavzi in the refrigerator at 2°C to 8°C. Do not freeze Hympavzi. Keep Hympavzi in the original carton to protect it from direct light.
- If necessary, Hympavzi can be stored at room temperature up to 30°C in the original carton for a maximum of 7 days. Do notuse Hympavzi if it has been out of the refrigerator for more than 7 days. Discard Hympavzi if it has been stored at room temperature for more than 7 days.
- Do notuse Hympavzi if it has passed the expiration date printed on the pre-filled pen.
- Keep Hympavzi and all medications out of the reach of children.
Materials Needed for Hympavzi Injection
Gather the following materials on a flat and clean surface:
- 1 Hympavzi pre-filled pen.
- 1 alcohol swab (not included).
- 1 cotton ball or gauze (not included).
- 1 sharps container for disposing of the pen (not included).
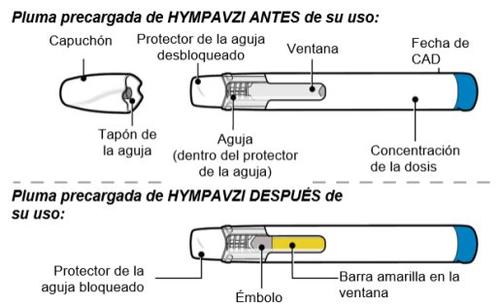
Preparation Steps
Step 1: Preparation
Removethe pen from the carton and keep it away from direct sunlight.
- Make surethat the name Hympavzi is on the carton and the pen label.
- Inspect the penfor visible damage, such as cracks or leaks.
- Wash and dryyour hands.
- Do not remove the cap until you are ready for injection.
- Discardthe pen if it is damaged or if the pen or carton has been dropped.
- Do notuse the pen if:
- it has been stored under direct light. Exposure to room light during dose preparation is acceptable.
- it has been frozen or thawed, or if it has been out of the refrigerator for more than 7 days.
- Do notshake the pen, as this may damage Hympavzi.
Note:To make the injection less painful, let the pen reach room temperature between 15 and 30 minutes inside the carton and away from direct sunlight.
Do notuse any other method to warm the pen, such as placing it in hot water or in a microwave.
Step 2: Check the Expiration Date (EXP)
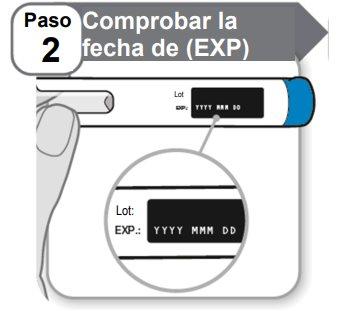
- Checkthe expiration date (EXP) printed on the pen label.
- Do notuse it if it has passed the expiration date.
Step 3: Check the Medication
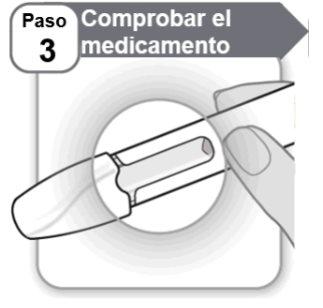
- Look carefullyat the medication through the pen window.
- It should be clear and colorless to light yellow.
- Do notuse the pen if the medication is cloudy, has a dark yellow color, or contains flakes or particles.
Note:It is normal to see a few air bubbles through the window.
If you have any questions about the medication, contact your doctor, nurse, or pharmacist.
Step 4: Choose and Clean the Injection Site
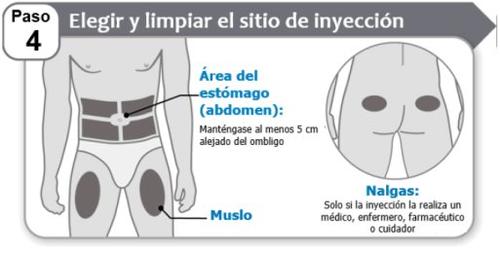
- Choosean injection site on the stomach area (abdomen) or thigh, unless your doctor, nurse, or pharmacist has told you to use a different site. Hympavzi can also be given in the buttocks, but only if the injection is given by a doctor, nurse, pharmacist, or caregiver. Stay at least 5 cm away from the navel.
- Alternatethe injection site each time you inject Hympavzi. You can use the same area of the body, but make sure to choose a different injection site within that area.
- Cleanthe injection site with soap and water, or with an alcohol swab if appropriate.
- Letthe area dry. Do nottouch, fan, or blow on the cleaned injection site.
- Do notinject Hympavzi into areas near bones or into areas where the skin is bruised, red, hard, or sore to the touch. Avoid injecting into areas with scars or stretch marks.
- Do notinject Hympavzi into a vein or muscle.
- Do notinject Hympavzi through clothing.
Step 5: Twist the Cap
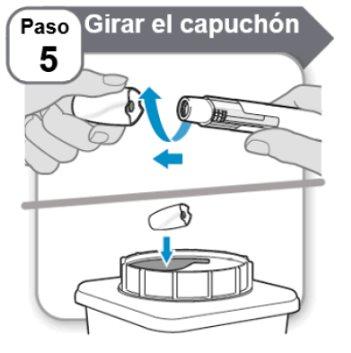
- Twistthe cap and pull it straight off.
- Placethe cap immediately in a sharps container. You will not need it again.
Note:
- It is normal to see a few drops of medication on the needle tip.
- The needle shield will remain inside the cap after it is removed.
Caution:Handle the pen with care, as it contains a needle.
Do notplace your hand over the needle shield or press your hand against it. Otherwise, you could injure yourself with the needle.
Injection Steps
Step 6: Inject the Medication
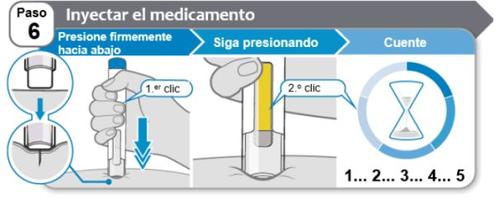
- Pressthe pen firmly downagainst the skin at a 90-degree angle and continue to pressuntil the injection is complete (step 7). You will hear the 1stclickwhen the injection starts.
- Continue to pressthe pen against the skin firmly while the yellow bar moves up the window. You will hear a 2nd clickwhen the injection is almostcomplete.
- After hearing the 2nd click, count slowly to 5to make sure you receive a full dose.
Do not remove the pen from the skin until you have counted slowly to 5 after hearing the 2nd click and until the yellow marker has filled the window completely.
Note:The needle is inserted into the skin when you press down. Your doctor, nurse, or pharmacist may suggest that you gently pinch the skin during the injection.
Note:If you do not hear a click when you press the pen against the skin, press down more firmly. If you still cannot start the injection, use a new Hympavzi pre-filled pen.
Caution:If you change your mind about the injection site after the needle is inserted into the skin, you must discard the pen and use a new Hympavzi pre-filled pen.
Step 7: Remove the Pen
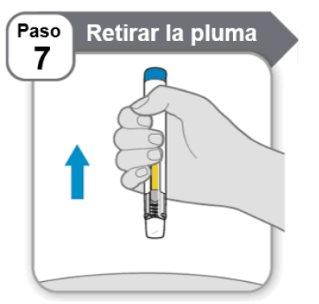
- Removethe pen from the skin.
- If you see a drop of medication on the skin, next time you inject, wait a little longer before removing the pen.
Note:After removing the pen from the skin, the needle will be automatically covered and the needle shield will lock.
The pen cannot be reused.
Step 8: Check the Window
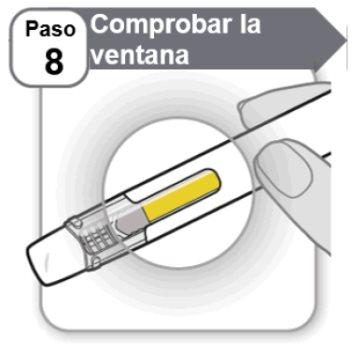
- Checkthe window to make sure that all of the medication has been injected.
If the yellow bar is not in the position shown, it means that you did not receive a full dose. Ask your doctor, nurse, or pharmacist for help.
Do not inject another dose.
Step 9: After Injection Care
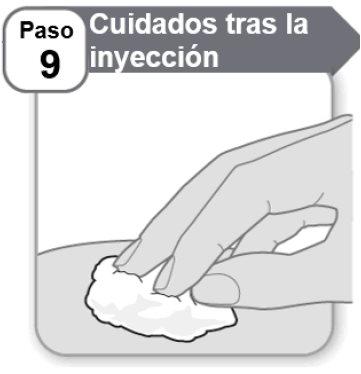
- Pressthe injection site gently for a few seconds with a clean cotton ball or gauze if you see a drop of blood.
- Do notrub the area.
Note:If the bleeding does not stop, contact your doctor, nurse, or pharmacist.
Step 10: Disposal
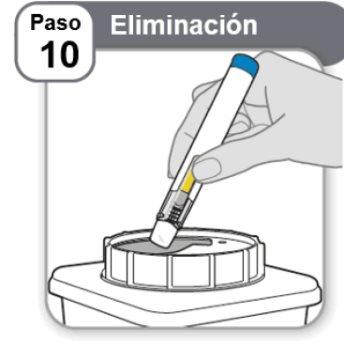
- Placethe used pen in a sharps container as instructed by your doctor, nurse, or pharmacist and in accordance with local health and safety regulations.
- Do notthrow away the pens in household trash.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HYMPAVZI 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PENDosage form: INJECTABLE, 100 mg/mlActive substance: concizumabManufacturer: Novo Nordisk A/SPrescription requiredDosage form: INJECTABLE, 100 mg/mlActive substance: concizumabManufacturer: Novo Nordisk A/SPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: concizumabManufacturer: Novo Nordisk A/SPrescription required
Online doctors for HYMPAVZI 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Discuss questions about HYMPAVZI 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions


















