HEXVIX 85 mg POWDER AND SOLVENT FOR INTRAVESICAL SOLUTION

How to use HEXVIX 85 mg POWDER AND SOLVENT FOR INTRAVESICAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
HEXVIX 85 mg powder and solvent for intravesical solution
Hexaminolevulinate
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience side effects, ask your doctor or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Hexvix and what is it used for
- What you need to know before you are given Hexvix
- How to use Hexvix
- Possible side effects
- Storage of Hexvix
- Package contents and further information.
1. What is Hexvix and what is it used for
This medicine is for diagnostic use only.
This medicine is used to help detect bladder cancer. It is given before your doctor uses a special device called a "cystoscope" that allows them to visualize the inside of your bladder. A cystoscope helps to visualize possible tumors and thus to eliminate abnormal cells, which glow in blue light after administration of Hexvix.
2. What you need to know before you are given HEXVIX
Do not use Hexvix:
- If you are allergic (hypersensitive) to the active substance or to any of the other ingredients of Hexvix, including the liquid used to dissolve it (see Section 6 Further information).
- If you have porphyria (a rare and hereditary blood disease)
Warnings and precautions:
Consult your doctor or nurse before you start using Hexvix.
- If you have a urinary tract infection or if you feel burning/stinging when urinating.
- If you have recently undergone BCG therapy in the bladder.
- If you have recently had bladder surgery.
These circumstances may cause local reactions in your bladder, which may make it difficult for your doctor to interpret what they see during the examination.
Other medicines and Hexvix:
Tell your doctor if you are taking or have recently taken other medicines, including those bought without a prescription.
Fertility, pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and using machines
Ask your doctor for advice about driving and using machines after taking Hexvix.
3. How to use Hexvix
Hexvix must be prepared and administered by qualified personnel. Hexvix is usually administered in a hospital or clinic.
Your doctor will give it to you in the following way:
- A small tube called a catheter will be inserted into your bladder.
- Your bladder will be emptied through this tube.
- Hexvix will be inserted into the bladder through this tube.
- Hexvix will remain inside your bladder for 60 minutes.
- After this time, the bladder will be emptied with the catheter.
- Your doctor will use a device called a cystoscope to examine your bladder.
If you use more Hexvix than you should
No side effects are expected to occur if the dwell time of Hexvix in the bladder is increased above 60 minutes or if the amount of Hexvix used is increased. If you are concerned about this, consult your doctor or nurse.
4. Possible side effects
Like all medicines, Hexvix can cause side effects, although not everybody gets them. There is a risk of possible side effects related to the technique (cystoscopy) used to examine your bladder. Generally, the use of Hexvix as a complementary method to standard cystoscopy for a more accurate diagnosis of bladder cancer is well tolerated. If side effects occur, they will be those typically associated with the examination technique, usually not serious or prolonged. The following side effects may occur after the examination procedure using this medicine:
Common(may affect up to 1 in 10 people):
- Feeling sick (nausea), vomiting.
- Diarrhea.
- Constipation.
- Muscle cramp or pain in and around the stomach (abdomen).
- Pain or difficulty urinating.
- Inability to empty the bladder (urinary retention).
- Blood in the urine.
- Pain after the examination (procedure).
- Fever (high temperature)
Uncommon(may affect up to 1 in 100 people):
- Headache
- Burning sensation when urinating (caused by infection or inflammation in the bladder).
- Frequent need to urinate.
- Blood poisoning (septicemia).
- Difficulty sleeping or insomnia.
- Pain in the tube called the urethra, through which urine passes.
- Continuous feeling of needing to urinate (and urgently).
- Increased white blood cell count, increased bilirubin (a yellow bile pigment) or liver enzyme concentrations, all of which should be observed in blood test results.
- Decrease in the number of red blood cells in the body (anemia).
- Inflammation of the glans penis (balanitis).
- Back pain.
- Gout.
- Rash (skin eruption).
- Itching (pruritus).
Frequency not known(cannot be estimated from the available data):
- Anaphylactoid shock (drop in blood pressure, increased heart rate, skin rash)
Reporting of side effects
If you experience any side effects, consult your doctor, even if they are not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Agency's website. www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hexvix
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer packaging. The expiry date refers to the last day of the month.
Powder and solvent: the product does not require special storage conditions.
Solution (after mixing): store between 2°C and 8°C (in the refrigerator) for a maximum period of 2 hours.
Hospital staff must ensure that the product is stored and disposed of properly and that it is not used after the expiry date printed on the outer packaging.
6. Package contents and further information
Composition of Hexvix
?The active substance is hexaminolevulinate in the form of hydrochloride.
?The other ingredients are disodium phosphate, potassium dihydrogen phosphate, sodium chloride, hydrochloric acid, sodium hydroxide, and water for injections
Appearance of the product and package contents
- Each package contains a vial of powder, white to beige or pale yellow in color, which contains 85 mg of the active substance hexaminolevulinate (as hydrochloride), and a pre-filled syringe, which contains 50 ml of a clear and colorless liquid for dissolving the powder.
- The Hexvix powder is dissolved in 50 ml of the solution provided in the package. Once the powder is mixed with the solvent, a solution is obtained that contains 1.7 mg/ml of hexaminolevulinate, which corresponds to 8 mmol/l of hexaminolevulinate solution.
Marketing authorization holder and manufacturer:
Photocure ASA
Hoffsveien 4
NO-0275 Oslo,
Norway
You can ask for more information about this medicine by contacting the marketing authorization holder.
This medicine has been authorized with the trade name Hexvix in the following European Economic Area member states and in the United Kingdom (Northern Ireland):Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Iceland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Slovenia, Spain, Sweden, and the United Kingdom (Northern Ireland).
Date of last revision of this package leaflet:January 2022
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
This information is intended only for healthcare professionals:
Handling instructions
Hexaminolevulinate may cause sensitization through skin contact.
All steps must be performed with sterile material and under aseptic conditions.
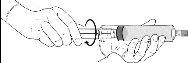
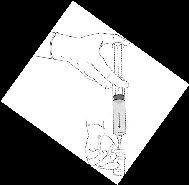
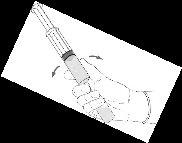
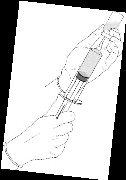
Reconstitution procedure: Hexvix powder and solvent for Hexvix in pre-filled syringe
|
|
|
|
|
|
|
|
|
|
Hexvix is now reconstituted and ready to use. The appearance of the reconstituted solution is a clear or slightly opalescent solution, and colorless to pale yellow.
Add two hours to the current time and write the resulting expiration time and date on the syringe label
This product is for single use only. Any remaining product should be discarded. No special requirements for disposal.
It has been demonstrated that the solution maintains its chemical and physical stability for a period of 2 hours at 2°C - 8°C. From a microbiological point of view, the product should be used immediately. If not, the storage times and conditions before use are the responsibility of the user and should not normally exceed 2 hours at 2°C - 8°C.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HEXVIX 85 mg POWDER AND SOLVENT FOR INTRAVESICAL SOLUTIONDosage form: INJECTABLE, 25 mg edrophonium bromide/2 mlActive substance: edrophoniumManufacturer: Mana Pharma S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.3%/0.3%/0.3% mol/molActive substance: Other diagnostic agentsManufacturer: Sociedad Española De Carburos Metalicos S.A.Prescription requiredDosage form: PULMONARY INHALATION, 0.28%/9.5% mol/molActive substance: Other diagnostic agentsManufacturer: Sociedad Española De Carburos Metalicos S.A.Prescription required
Online doctors for HEXVIX 85 mg POWDER AND SOLVENT FOR INTRAVESICAL SOLUTION
Discuss questions about HEXVIX 85 mg POWDER AND SOLVENT FOR INTRAVESICAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions