
HEMANGIOL 3.75 mg/ml ORAL SOLUTION

How to use HEMANGIOL 3.75 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
HEMANGIOL 3.75 mg/ml, Oral Solution
propranolol
Read all of this leaflet carefully before your child starts taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for your child only. Do not give it to other people, even if they have the same symptoms as your child, as it may harm them.
- If your child experiences any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is HEMANGIOL and what is it used for
- What you need to know before your child starts taking HEMANGIOL
- How to take HEMANGIOL to your child
- Possible side effects
- Storage of HEMANGIOL
- Package Contents and Additional Information
1. What is HEMANGIOL and what is it used for
What is HEMANGIOL
The name of your medicine is HEMANGIOL. The active substance is propranolol.
Propranolol belongs to a group of medicines known as beta-blockers.
What is it used for
This medicine is used to treat a disease called hemangioma. A hemangioma is an extra accumulation of blood vessels that have formed a lump in the skin or underneath it. The hemangioma can be superficial or deep. Sometimes it is called a "strawberry mark" because the surface of a hemangioma looks a bit like a strawberry.
Hemangiol is started in children who are between 5 weeks and 5 months old, when:
- the location and/or extent of the lesions put life or organ function at risk (they could affect vital organs or senses such as vision or hearing);
- the hemangioma is ulcerated (i.e., an open sore on the skin that does not heal) and is painful, and/or does not respond to basic wound care measures;
- there is a risk of permanent scarring or disfigurement.
2. What you need to know before your child starts taking HEMANGIOL
Do not use HEMANGIOL
If your child:
- was born prematurely and has not reached the corrected age of 5 weeks (the corrected age is the age the premature infant would have had if they had been born on the expected date);
- is allergic to propranolol or any of the other components of this medicine (listed in section 6). An allergic reaction can include a skin rash, itching, or difficulty breathing;
- has asthma or a history of breathing difficulties;
- has a slow heart rate for their age. You should consult with your doctor if you are unsure;
- has a heart problem (such as heart rhythm disorders and heart failure);
- has very low blood pressure;
- has circulatory problems that cause the fingers and toes to become numb and pale;
- has a tendency to have low blood sugar levels;
- has high blood pressure due to a tumor of the adrenal gland. This is called "pheochromocytoma".
If you are breastfeeding your child and if you are taking medications that should not be used with HEMANGIOL (see "If you are breastfeeding your child" and "Using HEMANGIOL with other medicines"), do not administerthis medicine to your child.
Warnings and Precautions
Before your child starts taking HEMANGIOL, tell your doctor:
- if your child has liver or kidney problems. This medicine is not recommended in case of liver or kidney failure;
- if your child has ever had an allergic reaction, regardless of its origin (e.g., medications or food substances, etc.). An allergic reaction can include a skin rash, itching, or difficulty breathing;
- if your child has psoriasis (a skin disease that produces red, dry patches of thickened skin), because this medicine may worsen the symptoms of this disease;
- if your child has diabetes: in this case, the frequency of blood glucose monitoring of your child should be increased.
- if your child has PHACE syndrome (a disorder that combines hemangioma and vascular malformations that can affect the cerebral blood vessels), because this medicine may increase the risk of stroke.
Important signs to look for after administering HEMANGIOL
Risk of Hypoglycemia
This medicine can mask the warning signs of hypoglycemia (also known as low blood sugar). It can also worsen hypoglycemia in children, especially during the fasting period (e.g., poor oral intake, infection, vomiting), when glucose demands increase (e.g., cold, stress, infections), or in case of overdose. These signs can be:
- Mild: paleness, fatigue, sweating, trembling, palpitations, anxiety, hunger, difficulty getting up.
- Severe: excessive sleepiness, difficulty responding, problems feeding, decreased body temperature, seizures (crisis), brief pauses in breathing, loss of consciousness.
The risk of developing hypoglycemia remains high throughout the treatment period.
To avoid the risks of hypoglycemia, you must administer HEMANGIOL during or immediately after a meal and avoid administering the last dose near bedtime (see section 3).You must feedyour child sufficiently andfrequently during treatment. If your child does not eat enough, if they are sick, or if they vomit, it is recommended to skip the dose. DO NOT ADMINISTER HEMANGIOL TO YOUR CHILD UNTIL THEY HAVE EATEN PROPERLY. If your child shows any signs of hypoglycemia while taking HEMANGIOL, stop treatment and call your doctor or go directly to the hospital immediately. If the child is conscious, give them an oral liquid containing sugar. |
Risk of Bronchospasm
Stop treatment and contact a doctor immediately if after administering HEMANGIOL to your child you observe the following symptoms indicative of bronchospasm (transient constriction of the bronchial tubes that causes difficulty breathing): cough, rapid or difficult breathing, or wheezing, with or without bluish skin color.
Stop treatment and contact a doctor immediately if your child has symptoms similar to a cold associated with difficulty breathing and/or wheezing while taking HEMANGIOL. |
Risk of Hypotension and Bradycardia (Slow Heart Rate)
HEMANGIOL may lower blood pressure (hypotension) and heart rate (bradycardia). For this reason, your child should be under close clinical and heart rate monitoring for 2 hours after the first intake and after a dose increase. Subsequently, during treatment, your doctor will perform periodic clinical examinations on your child.
Stop treatment and contact a doctor immediately if your child has any signs such as fatigue, feeling cold, paleness, bluish skin color, or fainting while taking HEMANGIOL. |
Risk of Hyperkalemia
HEMANGIOL may increase blood potassium levels (hyperkalemia). In the case of a large ulcerated hemangioma, your child's blood potassium levels should be measured.
If your child is going to receive general anesthesia
Tell your doctor that your child is taking HEMANGIOL. This is because your child may have low blood pressure if they are given certain anesthetics while taking this medicine (see "Using HEMANGIOL with other medicines"). It may be necessary to stop HEMANGIOL at least 48 hours before anesthesia.
If you are breastfeeding your child
- Tell your doctor before administering this medicine.
- Do not administer this medicine to your child if you are taking medications that should not be used with HEMANGIOL (see "Using HEMANGIOL with other medicines").
Using HEMANGIOL with other medicines
- Tell your doctor, pharmacist, or nurse if you are giving, have recently given, or might give any other medicine to your child. This is because HEMANGIOL may change the way other medicines work, and some medicines may affect the way HEMANGIOL works.
- Also, if you are breastfeeding your child, it is important that you tell your doctor, pharmacist, or nurse about the medicines you are taking, because they may pass into breast milk and interfere with your child's treatment. Your doctor will advise you whether it is necessary to stop breastfeeding or not.
In particular, if you are breastfeeding your child, tell your doctor or pharmacist if you or your child is taking:
- medicines for diabetes;
- medicines for heart and blood vessel problems, such as irregular heartbeats, chest pain, or high blood pressure;
- medicines for anxiety and depression, as well as more serious mental health problems, and epilepsy;
- medicines for treating tuberculosis;
- medicines for treating pain and inflammation;
- medicines used to lower blood fat levels;
- medicines used for anesthesia.
If you have any other questions, ask your doctor or pharmacist.
HEMANGIOL contains sodium and propylene glycol
This medicine contains less than 23 mg of sodium (1 mmol) per dose; this is essentially "sodium-free".
This medicine contains 2.08 mg of propylene glycol/kg/day. If the baby is less than 4 weeks old, consult your doctor or pharmacist, especially if the baby has been given other medicines that contain propylene glycol or alcohol.
3. How to administer HEMANGIOL to your child
Treatment of your child has been started by a doctor who has experience in the diagnosis, treatment, and management of infantile hemangioma.
Follow exactly the administration instructions for this medicine to your child as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Never modify the dose yourself that you are administering to your child. All dose increases and all adjustments for your child's weight must be made by your doctor.
Dose
- The dose is based on the weight of your child, following the scheme:
Weeks (daily dose) | Dose per intake | Intake times |
First week (1 mg/kg/day) | 0.5 mg/kg |
|
Second week (2 mg/kg/day) | 1 mg/kg | |
Third and subsequent weeks (3 mg/kg/day) | 1.5 mg/kg |
.
- When necessary, you can dilute the medicine in a small amount of infant milk or apple and/or orange juice suitable for the child's age, and administer it to your child in a bottle. Do not mix the medicine directly with a full bottle of milk or juice.
For children weighing up to 5 kg, you can mix the dose with a teaspoon of infant milk (approximately 5 ml). For children weighing more than 5 kg, you can mix the dose with a tablespoon of infant milk or fruit juice (approximately 15 ml).
Use the mixture within 2 hours of preparation.
How to administer HEMANGIOL to your child
- HEMANGIOL is for oral use.
- The medicine should be administered during your child's meal or immediately after it.
- The dose should always be measured with the oral syringe provided with the bottle.
- Administer HEMANGIOL directly into your child's mouth using the oral syringe included with the bottle.
- Feed your child frequently to avoid prolonged fasting.
- If your child does not eat or vomits, it is recommended to skip the dose.
- If your child vomits a dose, or if you are not sure if they have received all the medicine, do not give them another dose, simply wait until the next scheduled dose.
- The same person should administer HEMANGIOL and feed the child to avoid the risk of hypoglycemia. If different people are involved, it is essential to have good communication to ensure your child's safety.
Instructions for use:
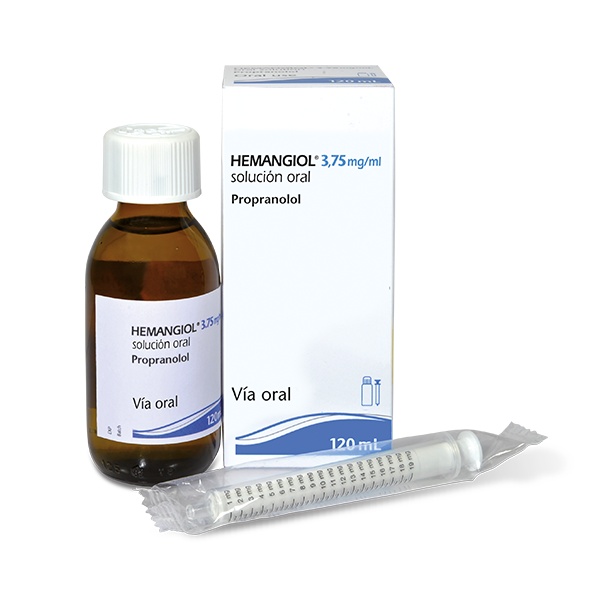
- Step 1. Extract the different elements from the box
The box contains the following elementsthat you will need to administer the medicine:
- The glass bottle containing 120 ml of propranolol oral solution.
- The oral dosing syringe in mg that is included with this medicine.
Extract the bottle and oral syringe from the box, and remove the syringe from the plastic bag.
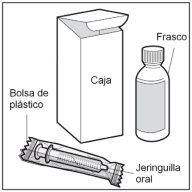
- Step 2. Check the dose
Check the dose of HEMANGIOL in milligrams (mg) as prescribed by your doctor.
Locate this number on the oral syringe.
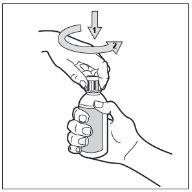
- Step 3. Open the bottle
The bottle has a child-resistant cap. This is how to open it: press the plastic cap down while turning it counterclockwise (to the left).
Do not shake the bottle before use.
- Step 4. Insert the syringe
Insert the tip of the oral syringe into the bottle in a vertical position, and push the piston all the way down.
Do not remove the syringe adapter from the mouth of the bottle.
Use only the oral syringe provided with the medicine to measure and administer the dose. Do not use a spoon or any other dispensing device.
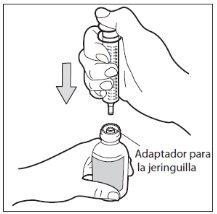
- Step 5: Withdraw the dose
With the oral syringe in place, turn the bottle upside down. Pull the piston of the syringe until you reach the number of mg you need.
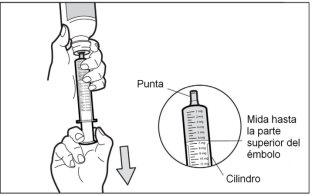
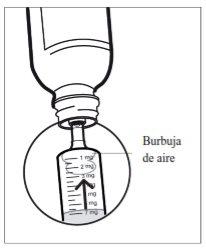
- Step 6: Check for air bubbles
If you see air bubbles in the syringe, hold the syringe vertically, push the piston up enough to completely expel all large air bubbles, and adjust it back to the prescribed dose.
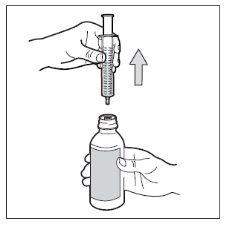
- Step 7. Remove the syringe
Turn the bottle to put it in a vertical position, and separate the syringe from the bottle. Be careful not to push the piston during this step.
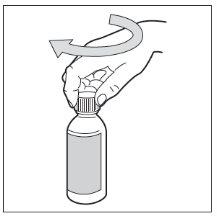
- Step 8. Close the bottle
Put the plastic cap back on the bottle by turning it clockwise (to the right).
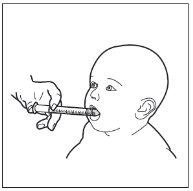
- Step 9. Administer HEMANGIOL to your child
Insert the syringe into your child's mouth and rest it on the inside of the cheek.
Now you can slowly expel HEMANGIOL from the syringe directly into your child's mouth.
Do not lay the child down immediately after administering the medicine.
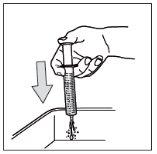
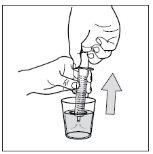
- Step 10: Clean the syringe.
Do not disassemble the syringe. Rinse the empty syringe with clean water after each use:
1- Take a glass of clean water
2- Pull the piston to the end
3- Throw the water into the sink
4- Repeat this cleaning process 3 times.
Do not use any product with soap or alcohol to clean it. Dry the outside with a cloth. Do not put the syringe in a sterilizer or dishwasher.
Store the bottle and syringe together in the box until the next use, in a safe place where the child cannot see or reach it. Discard the syringe when the bottle is empty.
If you administer more HEMANGIOL to your child than you should
If you have administered more HEMANGIOL to your child than you should, consult your doctor immediately.
If you forget to administer HEMANGIOL to your child
Miss the forgotten dose and do not administer a double dose to make up for the forgotten doses. Continue treatment with the usual frequency. One dose in the morning and one in the late afternoon.
If you interrupt your child's treatment with HEMANGIOL
HEMANGIOL may be stopped suddenly at the end of treatment, as decided by the doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
In addition, after the administration of HEMANGIOL, important warning signs of possible adverse effects such as low blood pressure, low heart rate, low blood sugar concentration, or bronchospasm (difficulty breathing) should be sought. See section 2 of this prospectus.
Adverse Effectsvery frequently (may affect more than 1 in 10 people):
- bronchitis (inflammation of the bronchi),
- sleep disorders (insomnia, poor quality sleep, and difficulty waking up),
- diarrhea and vomiting.
Adverse Effectsfrequently (may affect up to 1 in 10 people):
- bronchospasm (breathing difficulty),
- bronchiolitis (inflammation of small bronchi with breathing difficulty and wheezing in the chest, associated with cough and fever),
- decrease in blood pressure,
- decrease in appetite,
- agitation, nightmares, irritability,
- drowsiness,
- cold extremities,
- constipation, abdominal pain,
- erythema (skin redness).
- Diaper dermatitis.
Adverse Effectsinfrequently (may affect up to 1 in 100 people):
- conduction or cardiac rhythm disorders (slow or irregular heartbeats),
- urticaria (allergic skin reaction), alopecia (hair loss),
- decrease in blood sugar concentration,
- reduction in the number of leukocytes (white blood cells).
The frequency of the following adverse effects isunknown (cannot be estimated from the available data)
- seizures (crises) related to hypoglycemia (abnormally low glucose concentration in the blood),
- bradycardia (abnormally low heart rate),
- low blood pressure,
- very low leukocyte counts (white blood cells that fight infections),
- circulatory problems that cause fingers and toes to become numb and pale,
- elevation of potassium concentration in the blood.
Reporting of Adverse Effects
If your child experiences any type of adverse effect, consult your doctor or pharmacist, even if it is an adverse effect that is not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of HEMANGIOL
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and on the label of the vial. The expiration date is the last day of the month indicated.
Keep the vial in the outer packaging to protect it from light. Store the vial and the oral syringe in the case between each use. Do not freeze.
After the first opening of the vial, the medicine must be used within a maximum period of 2 months.
Medicines should not be thrown away through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Composition of HEMANGIOL
- The active ingredient is propranolol. Each ml contains 4.28 mg of propranolol hydrochloride, equivalent to 3.75 mg/ml of propranolol.
- The other ingredients are hydroxyethylcellulose, sodium saccharin, strawberry flavor (contains propylene glycol), vanilla flavor (contains propylene glycol), citric acid monohydrate, and purified water. For more information, see section 2 in "HEMANGIOL contains sodium and propylene glycol".
Appearance of HEMANGIOL and Package Contents
- HEMANGIOL is a clear, colorless or slightly yellowish oral solution with a fruity odor.
- It is supplied in a 120 ml amber glass bottle with a child-resistant screw cap. Package of 1 bottle.
- A polypropylene oral syringe graduated in mg of propranolol is supplied with each bottle.
Marketing Authorization Holder
PIERRE FABRE MEDICAMENT
Les Cauquillous
81500 Lavaur
FRANCE
Manufacturer
FARMEA
10 rue Bouché Thomas
ZAC Sud d’Orgemont
49000 ANGERS
FRANCE
Or
PIERRE FABRE MEDICAMENT PRODUCTION
Site PROGIPHARM, Rue du Lycée
45500 GIEN
FRANCE
You can request more information about this medicine by contacting the local representative of the marketing authorization holder.
Date of the Last Revision of this Prospectus:
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price225.59 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HEMANGIOL 3.75 mg/ml ORAL SOLUTIONDosage form: TABLET, 10 mgActive substance: propranololManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 40 mgActive substance: propranololManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 10 mgActive substance: propranololManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for HEMANGIOL 3.75 mg/ml ORAL SOLUTION
Discuss questions about HEMANGIOL 3.75 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











