
Propranolol Aurovitas
Ask a doctor about a prescription for Propranolol Aurovitas

How to use Propranolol Aurovitas
Leaflet accompanying the packaging: information for the user
Propranolol Aurovitas, 10 mg, coated tablets
Propranolol Aurovitas, 40 mg, coated tablets
Propranolol hydrochloride
You should read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Propranolol Aurovitas and what is it used for
- 2. Important information before taking Propranolol Aurovitas
- 3. How to take Propranolol Aurovitas
- 4. Possible side effects
- 5. How to store Propranolol Aurovitas
- 6. Contents of the packaging and other information
1. What is Propranolol Aurovitas and what is it used for
Propranolol Aurovitas contains propranolol hydrochloride, which belongs to a group of medicines called beta-blockers. It works on the cardiovascular system and other parts of the body.
Propranolol Aurovitas can be used:
- to treat high blood pressure (hypertension);
- to treat angina pectoris (chest pain);
- to treat certain arrhythmias (heart rhythm disorders);
- to protect the heart after a heart attack (myocardial infarction);
- to treat migraines;
- to treat essential tremor (involuntary and rhythmic shaking);
- to treat certain thyroid diseases (such as thyrotoxicosis, which is caused by an excess of thyroid hormones in the blood, and hyperthyroidism);
- to treat hypertrophic cardiomyopathy (thickening of the heart muscle);
- to treat pheochromocytoma (high blood pressure caused by a tumor, usually in the kidney area);
- for bleeding in the esophagus due to high blood pressure in the liver.
2. Important information before taking Propranolol Aurovitas
When not to take Propranolol Aurovitas
- if the patient is allergic to propranolol hydrochloride or any of the other ingredients of this medicine (listed in section 6),
- if the patient has untreated and/or uncontrolled heart failure,
- if the patient has been diagnosed with cardiogenic shock,
- if the patient has severe heart disease (second- or third-degree heart block) that may require a pacemaker,
- if the patient has conduction disorders or heart rhythm disorders,
- if the patient has very slow or very irregular heart rate,
- if the patient has increased blood acidity (metabolic acidosis),
- if the patient is on a strict diet,
- if the patient has asthma, wheezing, or other breathing disorders,
- if the patient has an untreated pheochromocytoma (high blood pressure caused by a tumor, usually in the kidney area),
- if the patient has severe circulatory disorders (which can cause tingling or paleness, or blue discoloration of fingers and toes),
- if the patient has squeezing chest pain at rest (Prinzmetal's angina),
- if the patient has very low blood pressure.
If any of the above warnings apply to the patient or if the patient is in doubt, they should consult a doctor before taking this medicine.
Warnings and precautions
Before starting treatment with Propranolol Aurovitas, the patient should discuss it with their doctor or pharmacist if:
- the patient has allergic reactions, e.g., to insect bites.
- the patient has diabetes, as Propranolol Aurovitas may change the normal response to low blood sugar levels, which usually include increased heart rate. Propranolol may cause low blood sugar levels even in patients without diabetes.
- the patient has thyrotoxicosis. Propranolol may mask the symptoms of thyrotoxicosis.
- the patient has kidney or liver disease (including liver cirrhosis). In such cases, the patient should consult a doctor, as certain control tests may be necessary during treatment.
- the patient has heart disease.
- the patient has muscle weakness (myasthenia).
- the patient has diseases such as chronic obstructive pulmonary disease and bronchospasm, as propranolol may worsen these conditions.
- the patient is taking calcium channel blockers with negative inotropic effects, e.g., verapamil and diltiazem (see "Propranolol Aurovitas and other medicines").
Propranolol Aurovitas and other medicines
The patient should tell their doctor or pharmacist about all medicines they are taking or have recently taken, as well as any medicines they plan to take. Propranolol Aurovitas may affect the action of certain other medicines, and some medicines may affect the action of Propranolol Aurovitas.
Propranolol Aurovitas should not be taken in combination with calcium channel blockers with negative inotropic effects (e.g., verapamil, diltiazem), as this may enhance their effects. This may cause severe hypotension and bradycardia.
Other medicines that should be used with caution in combination with Propranolol Aurovitas:
- nifedipine, nisoldipine, nicardipine, isradipine, lacidipine (used to treat hypertension or angina pectoris);
- lidocaine (a local anesthetic);
- disopyramide, quinidine, amiodarone, propafenone, and digitalis glycosides (used to treat heart diseases);
- adrenaline (a medicine that stimulates the heart);
- ibuprofen and indomethacin (used for pain and inflammation);
- ergotamine, dihydroergotamine, or rizatriptan (used to treat migraines);
- chlorpromazine and thioridazine (used to treat certain mental disorders);
- cimetidine (used to treat stomach disorders);
- rifampicin (used to treat tuberculosis);
- theophylline (used to treat asthma);
- warfarin (a blood thinner) and hydralazine (used to treat hypertension);
- fingolimod (used to treat multiple sclerosis);
- fluvoxamine and barbiturates (used to treat anxiety and insomnia);
- monoamine oxidase inhibitors (used to treat depression).
If the patient is taking clonidine (used to treat hypertension or migraines) and propranolol, they should not stop taking clonidine without their doctor's advice. If clonidine needs to be discontinued, the doctor will inform the patient how to do it.
Propranolol Aurovitas with food, drink, and alcohol
Alcohol may affect the action of the medicine.
Surgical procedures
If the patient is scheduled for surgery, they should inform the anesthesiologist or medical staff that they are taking Propranolol Aurovitas.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before taking this medicine.
Pregnancy:
This medicine should not be used during pregnancy unless the doctor considers it necessary.
Breastfeeding:
This medicine should not be used during breastfeeding.
Driving and using machines
It is unlikely that the medicine will affect the ability to drive or use machines. However, some people may occasionally experience dizziness or fatigue while taking propranolol. If such symptoms occur, the patient should consult a doctor for advice.
Important information about some ingredients of Propranolol Aurovitas
Propranolol Aurovitas contains lactose. If the patient has been diagnosed with intolerance to some sugars, they should contact their doctor before taking the medicine.
Propranolol Aurovitas contains sodium. This medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means it is considered "sodium-free".
3. How to take Propranolol Aurovitas
This medicine should always be taken exactly as advised by the doctor or pharmacist. If the patient is in doubt, they should consult a doctor or pharmacist.
Regarding the 40 mg dose:
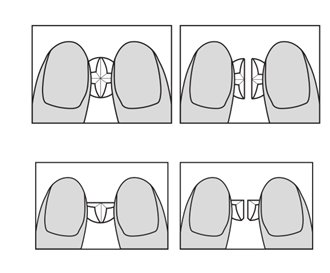
If the doctor has prescribed 1/4 (a quarter) of a tablet or 1/2 (half) of a tablet, Propranolol Aurovitas should be broken in the following way:
Figure 1 and 2
Figure 3 and 4
- Place the tablet with the dividing line facing up on a flat, rigid, and clean surface (e.g., a table or work surface).
- Place the index fingers along one of the dividing lines and press down to break the tablet (see Figure 1 and 2).
- To obtain 1/4 (a quarter) of a tablet, repeat the action from 1/2 (half) of a tablet (see Figure 3 and
- 4).
Propranolol Aurovitas tablets should be taken before a meal, with a small amount of water. They should not be chewed.
The patient should not stop taking the medicine without their doctor's advice.
Adults
The recommended dosage for an adult is as follows:
| Recommended dose | Total daily dose (maximum) | |
| Hypertension (high blood pressure) | Initially 40 mg two or three times a day; the doctor may increase the dose by 80 mg per day at weekly intervals | 160 mg to 320 mg |
| Angina pectoris (chest pain) and tremors | Initially 40 mg two or three times a day; the doctor may increase the dose by 40 mg per day at weekly intervals | 120 mg to 240 mg |
| Heart protection after myocardial infarction | Initially 40 mg four times a day, and after a few days 80 mg twice a day | 160 mg |
| Migraine | Initially 40 mg two or three times a day; the doctor may increase the dose by 40 mg per day at weekly intervals | 80 mg to 160 mg |
| Arrhythmias, certain thyroid diseases (hyperthyroidism and thyrotoxicosis), and hypertrophic cardiomyopathy | 10 to 40 mg three or four times a day | 120 mg to 160 mg |
| (thickening of the heart muscle) | ||
| Pheochromocytoma | Before surgery: 60 mg per day Treatment for inoperable tumor: 30 mg per day | 30 mg to 60 mg |
| Liver disease with high blood pressure in the liver | Initially 40 mg twice a day; then the doctor will increase the dose to 80 mg twice a day | 160 mg to 320 mg |
Children and adolescents
Propranolol Aurovitas may also be used to treat children with migraines and arrhythmias:
- In the treatment of migraines, the dose for children under 12 years old is 20 mg, two or three times a day, and for children over 12 years old, the dose is the same as for adults.
- In the treatment of arrhythmias, the dose of the medicine will be adjusted by the doctor according to the child's age or body weight.
Elderly patients
In elderly patients, the dosage should be started with the lowest dose. The optimal dose will be determined by the doctor individually for each patient.
Renal or hepatic impairment
The optimal dose will be determined by the doctor individually for each patient.
Taking a higher dose of Propranolol Aurovitas than recommended
In case of accidental ingestion of a higher dose than recommended, the patient should immediately go to the emergency department of the nearest hospital or contact a doctor or pharmacist. Symptoms of overdose include very slow heart rate, significantly low blood pressure, heart failure, and breathing difficulties, with accompanying symptoms such as fatigue, hallucinations, tremors, disorientation, nausea, vomiting, muscle cramps, fainting, or coma, low blood sugar levels. The patient should always bring the remaining tablets and packaging to allow for identification of the medicine.
Missing a dose of Propranolol Aurovitas
If the patient forgot to take the medicine at the usual time, they should take it as soon as they remember. However, if it is almost time for the next dose, they should skip the missed dose.
The patient should not take a double dose to make up for the missed dose.
Stopping treatment with Propranolol Aurovitas
The patient should not stop taking the medicine without consulting a doctor. In some cases, it may be necessary to gradually discontinue the medicine.
If the patient has any further doubts about taking this medicine, they should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur during treatment with this medicine.
Common (may affect up to 1 in 10 people):
- cold fingers and toes;
- slow heart rate;
- numbness and cramps in the fingers, followed by a feeling of warmth and pain (Raynaud's syndrome);
- sleep disturbances and/or nightmares;
- fatigue;
- shortness of breath.
Uncommon (may affect up to 1 in 100 people):
- nausea, vomiting, and diarrhea.
Rare (may affect up to 1 in 1,000 people):
- worsening of breathing difficulties, sometimes fatal, if the patient has or has had asthma or asthma-like disorders;
- heart failure, worsening of heart disease;
- swelling of the face, tongue, larynx, abdomen, or hands and feet (angioedema);
- dizziness, especially when standing up;
- worsening of blood circulation in patients with existing circulatory disorders;
- hair loss (excessive hair loss);
- mood changes;
- disorientation;
- memory loss;
- psychosis or hallucinations (mental disorders);
- paresthesia (abnormal sensations, usually tingling or prickling);
- vision disturbances;
- dry eyes;
- rash, including worsening of psoriasis;
- blood disorders, such as decreased platelet count (thrombocytopenia), with easy bruising and bleeding;
- purpura (purple spots on the skin).
Very rare (may affect up to 1 in 10,000 people):
- severe muscle weakness (myasthenia);
- low blood sugar levels, which may occur in both diabetic and non-diabetic patients, including newborns, infants, and children, elderly patients, patients undergoing hemodialysis, or patients taking anti-diabetic medicines; it may also occur in patients who are fasting or have recently fasted, as well as in patients with chronic liver disease;
- excessive sweating.
Frequency not known (frequency cannot be estimated from the available data):
- headache or seizures related to low blood sugar levels;
- impotence (inability to achieve an erection);
- decreased blood flow to the kidneys;
- joint pain (arthralgia);
- constipation;
- dry mouth;
- shallow breathing or shortness of breath (dyspnea);
- conjunctivitis (red eye);
- depression;
- lack of white blood cells (agranulocytosis);
- worsening of angina pectoris (chest pain).
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, it is possible to gather more information on the safety of the medicine.
5. How to store Propranolol Aurovitas
The medicine should be stored out of sight and reach of children.
There are no special precautions for storing the medicine.
The medicine should not be used after the expiration date stated on the blister pack and carton after: EXP.
The expiration date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Propranolol Aurovitas contains
- The active substance of the medicine is propranolol hydrochloride. Each coated tablet contains 10 mg or 40 mg of propranolol hydrochloride.
- The other ingredients are: Core:microcrystalline cellulose (type 101), lactose monohydrate, corn starch, sodium carboxymethylcellulose (type A), povidone (K 30), magnesium stearate Coating:hypromellose 2910 (E 464), macrogol 6000 (E 1521), titanium dioxide (E 171)
What Propranolol Aurovitas looks like and contents of the pack
Coated tablet.
Propranolol Aurovitas, 10 mg:
White or almost white, round, biconvex coated tablets with the inscription "I" on one side and "10" on the other. The diameter of the tablet is 5.0 mm.
Propranolol Aurovitas, 40 mg:
White or almost white, round, biconvex coated tablets with the inscription "I 40" on one side and a dividing line on the other. The diameter of the tablet is 8.0 mm.
The tablet can be divided into equal doses.
The medicine is packaged in PVC/Aluminum blisters.
Pack sizes: 20, 28, 30, 50, 56, 60, 90, 98, and 100 coated tablets.
Not all pack sizes may be marketed.
Marketing authorization holder:
Aurovitas Pharma Polska Sp. z o.o.
ul. Sokratesa 13D lokal 27
01-909 Warsaw
e-mail: [email protected]
Manufacturer/Importer:
APL Swift Services (Malta) Ltd
HF26, Hal Far Industrial Estate, Hal Far
Birzebbugia, BBG 3000
Malta
Generis Farmacêutica, S.A.
Rua João de Deus, 19
2700-487 Amadora
Portugal
Arrow Generiques
26 Avenue Tony Garnier
69007 Lyon
France
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium:
Propranolol AB 40 mg film-coated tablets.
France:
PROPRANOLOL ARROW 40 mg, scored film-coated tablet
Germany:
Propranolol PUREN 10 mg film-coated tablets / Propranolol PUREN 40 mg film-coated tablets
Netherlands:
Propranolol HCl Aurobindo 10 mg, film-coated tablets / Propranolol HCl Aurobindo 40 mg, film-coated tablets
Poland:
Propranolol Aurovitas
Portugal:
Propranolol Generis
Spain:
Propranolol Aurovitas 10 mg film-coated tablets EFG
Propranolol Aurovitas 40 mg film-coated tablets EFG
Date of last revision of the leaflet: 03/2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAPL Swift Services (Malta) Ltd. Arrow Generiques - Lyon Generis Farmacêutica, S.A.,
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Propranolol AurovitasDosage form: Tablets, 10 mgActive substance: propranololPrescription requiredDosage form: Tablets, 40 mgActive substance: propranololPrescription requiredDosage form: Tablets, 10 mgActive substance: propranololPrescription required
Alternatives to Propranolol Aurovitas in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Propranolol Aurovitas in Espanha
Alternative to Propranolol Aurovitas in Ukraine
Online doctors for Propranolol Aurovitas
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Propranolol Aurovitas – subject to medical assessment and local rules.










