
GELASPAN 40 mg/ml SOLUTION FOR INFUSION

How to use GELASPAN 40 mg/ml SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Gelaspan 40 mg/ml solution for infusion
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.,it is important that you are aware of the following information before starting treatment with this medicine..
|
Contents of the package leaflet:
- What is Gelaspan and what is it used for
- What you need to know before you use Gelaspan
- How to use Gelaspan
- Possible side effects
- Storage of Gelaspan
Contents of the pack and other information
1. What is Gelaspan and what is it used for
Gelaspan is one of the so-called plasma substitutes. This means that it replaces the fluid lost from the blood vessels.
Gelaspan is used to:
- Replace blood and body fluids that have been lost after, for example, an operation, an accident or a burn. It can be combined with blood transfusions if necessary.
- Prevent low blood pressure (hypotension) that can occur when you receive spinal or epidural anesthesia or due to severe and imminent blood loss in a surgical setting.
Fill the circulating blood volume during the use of, for example, an extracorporeal circulation system with other perfusion fluids.
2. What you need to know before you use Gelaspan
Do not use Gelaspan
- if you are allergic to gelatin or any of the other components of this medicine (listed in section 6)
- if you are allergic to an allergen called “galactosa-α-1,3-galactosa (alpha-Gal)” or to red meat (mammalian meat) and offal
- if your circulating blood volume is too high
- if you have too much water in your body
- if you have certain types of heart failure (acute congestive heart failure)
- if you have an abnormally high potassium level in your blood.
Warnings and precautions
Consult your doctor or pharmacist or nurse before starting treatment with Gelaspan.
Tell your doctor
- if you have an allergic disease such as asthma, as you may be at greater risk of experiencing an allergic reaction
- in these cases, Gelaspan should not be administered to you due to possible cross-reactions:
- if you know you are allergic to red meat (mammalian meat) or offal
- if you have tested positive for antibodies (IgE) against the alpha-Gal allergen
Your doctor will be particularly careful with your situation if you suffer from
- heart problems
- high blood pressure
- fluid in your lungs
- severe kidney problems.
Administering large amounts of fluid through an intravenous drip can worsen your condition.
Your doctor will also act with caution
- if you have a severe increase in sodium or chloride in the blood
- if you retain water and salt, which may be associated with tissue inflammation.
- if you have too much potassium in the blood or if you are taking or receiving medications that cause potassium retention
- if your blood clotting is severely affected
- if you are an elderly person
While you are receiving Gelaspan, your blood composition will be monitored. If necessary, your doctor may give you other medications such as salts and fluids.
Children:
There is limited experience with the use of Gelaspan in children. The doctor will only administer this medicine to children when they consider it absolutely necessary.
Laboratory test results
Your doctor may take blood or urine samples before administering Gelaspan to you. This is because some laboratory test results may be affected after receiving this medicine and are therefore not reliable.
Other medicines and Gelaspan
Tell your doctor or pharmacist if you are taking or using or have recently taken or used any other medicines, including those obtained without a prescription.
In particular, your doctor should know if you are taking or receiving medications that make you retain sodium (such as spironolactone, triamterene, amiloride; ACE inhibitors such as captopril or enalapril, corticosteroids such as cortisone or non-steroidal anti-inflammatory drugs such as diclofenac), concomitant administration with this medicine may cause swelling of the arms, hands, legs, and feet (edema). Also, inform your doctor if you are taking medications that make you lose potassium, such as medications that increase water loss.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking any other medicine.
Pregnancy
If you are pregnant, inform your doctor. Due to the possibility of allergic reactions, the use of this medicine should be avoided during pregnancy. However, your doctor may administer this medicine in emergency situations.
Breastfeeding
If you are breastfeeding, inform your doctor. There is limited information on the excretion of this medicine in breast milk. Your doctor will decide whether it is necessary to interrupt breastfeeding or interrupt treatment with this medicine after considering the benefit of breastfeeding for the child and the benefit of treatment for you.
Fertility
No data are available on the effect of this medicine on human or animal fertility. However, due to the nature of its components, it is considered unlikely to affect fertility.
Driving and using machines
The medicine does not affect the ability to drive or use machines.
3. How to use Gelaspan
Your doctor will only administer Gelaspan to you if they consider that other products called crystalloids are not sufficient on their own.
Your doctor will carefully adjust the dose of Gelaspan to prevent fluid overload. This will be done especially if you have problems with your lungs, heart, or circulation.
Dosage
Gelaspan is administered intravenously, i.e., drop by drop.
Adults
The amount of fluid administered and the duration of treatment will depend on the amount of blood or fluid lost and your condition.
Your doctor will perform tests (e.g., for blood pressure and blood) during treatment and adjust the dose of Gelaspan according to your needs. If necessary, you may also receive blood or hematite concentrates.
Use in children
There is limited experience with the use of this medicine in children. The doctor will only administer this medicine if they consider it essential for the child's recovery. In these cases, the clinical condition will be taken into account and the treatment will be monitored with special care.
If you have received more Gelaspan than you should
An overdose of Gelaspan can cause too high a blood volume (hypervolemia) and fluid overload that can affect the heart and lung function.
If an overdose occurs, your doctor will administer the necessary treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Gelaspan can cause side effects, although not everybody gets them.
All plasma substitutes carry a slight risk of allergic reactions, usually mild or moderate, but in very rare cases, they can also be severe. It is assumed that these reactions are more frequent in patients with known allergic disorders, such as asthma. For this reason, you will be closely monitored by a healthcare professional, especially at the start of the infusion.
The following side effects may be serious. If you experience any of the following side effects, consult a doctor immediately:
Rare (may affect up to 1 in 1,000 people):
Allergic reactions (anaphylactic/anaphylactoid) including, for example, difficulty breathing, wheezing, nausea, vomiting, dizziness, sweating, chest or throat tightness, stomach pain, swelling of the neck and face.
If an allergic reaction occurs, the infusion will be stopped immediately and you will be given the necessary treatment (see also section 2 "What you need to know before you use Gelaspan", especially for allergies involving the allergens called galactosa-α-1,3-galactosa (alpha-Gal), red meat, and offal).
Other side effects include:
Very common (may affect up to 1 in 10 people):
- decrease in red blood cells and blood proteins
Common (may affect up to 1 in 100 people):
- it is possible that your blood will not clot as well as before and that you may notice that you bleed more easily
Very rare (may affect up to 1 in 10,000 people):
- rapid heartbeat
- low blood pressure
- fever, chills.
Frequency not known (cannot be estimated from the available data):
- nausea, vomiting, stomach pain
- decrease in oxygen in the blood that can make you feel dizzy
Other side effects in children
No data are available on differences in side effects in children.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency website www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Gelaspan
Keep this medicine out of the sight and reach of children.
Do not use Gelaspan after the expiry date which is stated on the label and carton. The expiry date refers to the last day of the month stated.
Do not store above 25°C. Do not freeze.
Do not use Gelaspan if you notice:
- turbidity or discoloration of the solution
- leakage from the container.
Gelaspan that has been opened or partially used should be discarded. Partially used vials or bags should not be reconnected.
6. Contents of the pack and other information
Composition of Gelaspan
Active substances:
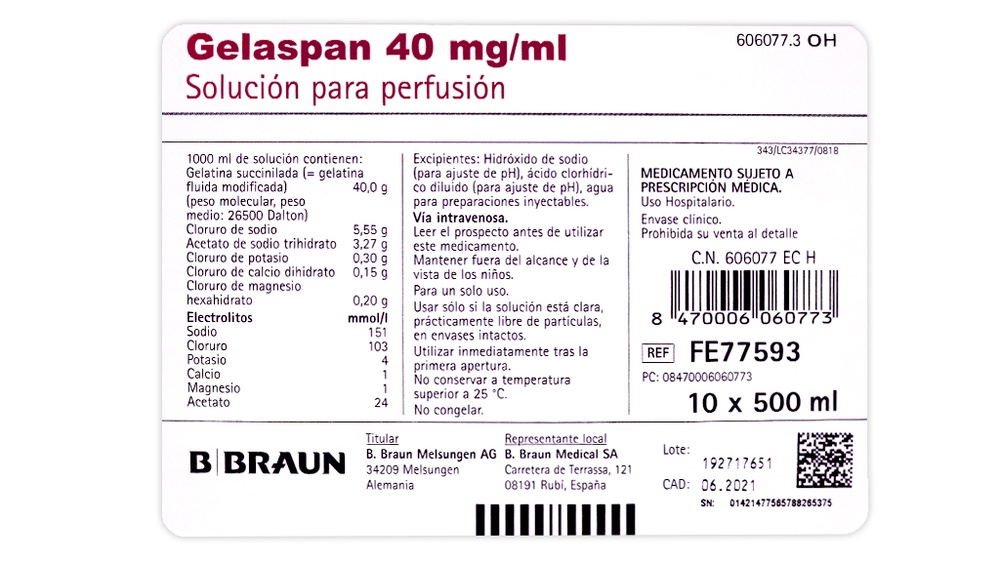
1,000 ml of solution contain:
Succinylated gelatin (modified fluid) 40.0 g
Sodium chloride 5.55 g
Sodium acetate trihydrate 3.27 g
Potassium chloride 0.30 g
Calcium chloride dihydrate 0.15 g
Magnesium chloride hexahydrate 0.20 g
Electrolyte concentration
Sodium 151 mmol/l
Chloride 103 mmol/l
Potassium 4 mmol/l
Calcium 1 mmol/l
Magnesium 1 mmol/l
Acetate 24 mmol/l
The other ingredients are:
Water for injections, diluted hydrochloric acid (for pH adjustment), and sodium hydroxide (for pH adjustment).
Appearance of the product and pack contents
Gelaspan is a solution for infusion administered by intravenous drip (drip into a vein).
It is a sterile, clear, and colorless or slightly yellowish solution.
Gelaspan is available in:
- Polyethylene vials "Ecoflac plus" containing 500 ml, available in packs of 10 x 500 ml
- "Ecobag" plastic bags (without PVC), sealed with rubber stoppers, containing 500 ml, available in packs of 20 x 500 ml.
Not all pack sizes may be marketed.
Marketing authorisation holder, manufacturer, and local representative
Marketing authorisation holder and manufacturer
- Braun Melsungen AG
Carl-Braun-Straße 1
34212 Melsungen, Germany
Postal address
34209 Melsungen, Germany
Phone: +49/5661/71-0
Fax: +49/5661/71-4567
Batch release manufacturer in the UK
- Braun Medical Limited
Brookdale Road
Thorncliffe Park Estate
Chapeltown
Sheffield
S35 2PW
United Kingdom
Batch release manufacturer in ES and PT
- Braun Medical S.A.
Carretera de Terrassa, 121
08191 Rubí (Barcelona)
Spain
You can obtain further information on this medicine from the local representative of the marketing authorisation holder
- Braun Medical, SA
Ctra. Terrassa, 121
08191 Rubí (Spain)
This medicine has been authorised in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Member State | Medicine name |
Austria | Gelofusin Iso 40 mg/ml Infusionslösung |
Belgium | Isogelo oplossing voor infusie, solution pour perfusion, Infusionslösung |
Bulgaria | Gelofusine Balance 4% solution for Infusion |
Czech Republic | Gelaspan 4% |
Denmark | Gelaspan |
Estonia | Gelaspan infusioonilahus 4% |
France | Gelaspan, solution pour perfusion |
Germany | Gelafundin ISO 40 mg/ml Infusionslösung |
Greece | Gelaspan solution for Infusion 4% |
Hungary | Gelaspan 4% oldatos infúzió |
Ireland | Gelaspan Solution for Infusion |
Italy | Gelaspan |
Latvia | Gelaspan 4% Solution for Infusion |
Lithuania | Gelaspan 4% infuzinis tirpalas |
Luxembourg | Gelafundin ISO 40 mg/ml Infusionslösung |
Malta | Gelaspan 4% Solution for Infusion |
Netherlands | Gelaspan, oplossing voor infusie |
Norway | Gelaspan |
Poland | Gelaspan |
Portugal | Gelaspan |
Romania | Gelaspan 40 mg/ml solutie perfuzabila |
Slovakia | Gelaspan 4% |
Slovenia | Gelaspan 40 mg/ml raztopina za infundiranje |
Spain | Gelaspan 40 mg/ml solution for infusion |
Sweden | Gelaspan |
United Kingdom (Northern Ireland) | Gelaspan solution for infusion |
Date of last revision of this leaflet:April 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products http://www.aemps.gob.es/
This information is intended only for healthcare professionals:
Precautions for use
Gelaspan should not be infused through the same infusion line with blood, blood components, or blood derivatives (red blood cell concentrates, plasma, and plasma fractions).
During compensation for severe blood loss by infusing large amounts of Gelaspan, the hematocrit and electrolytes should be monitored. The hematocrit should not decrease below 25%. In elderly or severely ill patients, it should not be lower than 30%.
Similarly, the dilution effect on clotting factors should be observed in these situations, especially in patients with existing hemostasis disorders.
Since the product does not replace losses of plasma proteins, it is recommended to monitor plasma protein concentrations.
In acute emergency situations, Gelaspan can be infused rapidly by pressure infusion (500 ml can be administered in 5-10 minutes) until the signs of hypovolemia are relieved.
Before rapid infusion, Gelaspan can be warmed to no more than 37°C.
In the case of pressure infusion, which may be necessary in life-threatening emergency situations, all air should be removed from the container and infusion equipment before administering the solution to avoid the risk of gas embolism that could be associated with infusion.
Influence on laboratory tests
Blood laboratory tests (blood group and irregular antibodies) are possible after Gelaspan infusions. However, it is recommended to take blood samples before Gelaspan infusion to avoid a doubtful interpretation of the results.
Gelaspan may influence the following chemical-clinical tests, resulting in falsely elevated values:
- erythrocyte sedimentation rate
- urine specific gravity
- non-specific protein tests, for example, the Biuret method.
Incompatibilities
In the absence of compatibility studies, this medicine should not be mixed with other medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GELASPAN 40 mg/ml SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 3 g; 0.5382 g; 0.0305 g; 0.0373 g; 0.3360 gActive substance: gelatin agentsManufacturer: Fresenius Kabi España, S.A.U.Prescription requiredDosage form: INJECTABLE PERFUSION, 200 g/lActive substance: albuminManufacturer: Biotest Pharma GmbhPrescription requiredDosage form: INJECTABLE PERFUSION, 200 g/lActive substance: albuminManufacturer: Csl Behring GmbhPrescription required
Online doctors for GELASPAN 40 mg/ml SOLUTION FOR INFUSION
Discuss questions about GELASPAN 40 mg/ml SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















