
GAMMAGARD S/D 10 G, POWDER AND SOLVENT FOR PERFUSION SOLUTION

How to use GAMMAGARD S/D 10 G, POWDER AND SOLVENT FOR PERFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
GAMMAGARD S/D 10 g, Powder and Solvent for Solution for Infusion
Human Normal Immunoglobulin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What GAMMAGARD S/D is and what it is used for
- What you need to know before you use GAMMAGARD S/D
- How to use GAMMAGARD S/D
- Possible side effects
- Storage of GAMMAGARD S/D
- Contents of the pack and other information
1. What GAMMAGARD S/D is and what it is used for
GAMMAGARD S/D belongs to a group of medicines called immunoglobulins. These medicines contain human antibodies, which are also present in blood. Antibodies help to fight infections. Medicines like GAMMAGARD S/D are used when you do not have enough antibodies in your blood. These patients often suffer from frequent infections. GAMMAGARD S/D can also be used when additional antibodies are needed to treat certain inflammatory disorders (autoimmune diseases).
GAMMAGARD S/D 10 g is used for
Treatment of patients who do not have enough antibodies (replacement therapy). There are five groups:
- Patients with a congenital lack of antibody production (primary immunodeficiency syndromes (PID)) such as:
- congenital agammaglobulinemia or hypogammaglobulinemia,
- common variable immunodeficiency,
- severe combined immunodeficiencies,
- Wiskott-Aldrich syndrome
- Patients with a blood cancer (chronic lymphocytic leukemia) that causes a lack of antibody production and recurrent infections when preventive treatment with antibiotics has failed.
3 Patients with a bone marrow cancer (multiple myeloma) and a lack of antibody production with recurrent infections in whom the response to vaccination against certain bacteria (pneumococci) has failed.
4 Children and adolescents (0 to 18 years) with congenital AIDS and frequent infections.
5 Patients with low antibody production after a bone marrow cell transplant from another person.
Treatment of patients with certain inflammatory disorders (immunomodulatory effect). There are three groups:
- Patients who do not have enough platelets in their blood (idiopathic/primary thrombocytopenic purpura, ITP) and are at high risk of bleeding or are about to undergo surgery.
- Patients with a disease that causes inflammation of multiple organs in the body (Kawasaki disease).
- Patients with a disease characterized by inflammation of the nerves throughout the body (Guillain-Barré syndrome).
2. What you need to know before you use GAMMAGARD S/D
Do not useGAMMAGARDS/D
- If you are allergic (hypersensitive) to immunoglobulins or to any of the other components of this medicine (listed in section 6).
- If you have an immunoglobulin A deficiency. You may have anti-immunoglobulin A antibodies in your blood. GAMMAGARD S/D contains very small amounts of immunoglobulin A and you may develop an allergic reaction.
Warnings and precautions
Monitoring period required during infusion
- You will be closely monitored during the GAMMAGARD S/D infusion period to avoid an allergic reaction. Your doctor will ensure that the infusion rate of GAMMAGARD S/D is suitable for you.
There may be a higher risk of side effects:
- if GAMMAGARD S/D is administered at a high rate,
- if you have a disorder characterized by low levels of antibodies in the blood (hypo- or agammaglobulinemia),
- if you have not received this medicine before or
- if a long period (e.g. several weeks) has passed since you last received it.
In these cases, you will be closely monitored during the infusion and for one hour after the infusion has finished, as there may be a higher risk of side effects.
If you have recently received GAMMAGARD S/D, you will only be observed during the infusion and for at least 20 minutes after the infusion.
When to stop or reduce the infusion rate
In rare cases, your body may be sensitized to medicines that contain antibodies. This can occur especially if you have an immunoglobulin A deficiency. In these rare cases, you may experience allergic reactions such as a sudden drop in blood pressure or shock, even if you have previously received treatment with medicines that contain antibodies.
- If you notice any of the following symptoms, inform your doctor or nurse immediately:
- Sudden wheezing, difficulty breathing or chest tightness
- Headache
- Fever
- Swelling of the eyelids, face, lips or blood vessels
- Hives or itchy skin rashes
- Itching all over the body
Depending on your doctor's decision, the infusion rate may be reduced or stopped.
Special patient groups
Your doctor should exercise caution if you are overweight, elderly, diabetic, immobilized, using estrogens, have a permanent vascular catheter or are prone to thrombosis.
Your doctor will closely monitor you if you have:
- high blood pressure
- low blood volume (hypovolemia)
- increased blood viscosity or vascular problems (vascular diseases including cardiac output or thrombotic episodes)
- excessive blood clotting or coagulation disorders.
In these cases, immunoglobulins may increase the risk of myocardial infarction, stroke, pulmonary embolism or deep vein thrombosis, although this is very rare.
Tell your doctor if you are diabetic.
This medicine contains glucose. GAMMAGARD S/D does not contain sucrose or maltose.
Patients with diabetes mellitus should be aware that a 5% (50 mg/ml) solution of GAMMAGARD S/D contains 400 mg of glucose per gram of IgG. A 70 kg patient who receives a dose of 1 g/kg of IgG would receive 28 grams of glucose or 112 calories. This may affect their blood sugar levels.
Your doctor will also exercise caution
- if you have or have had kidney problems
- if you are taking medicines that may harm your kidneys (nephrotoxic medicines), as there is a very rare possibility of acute kidney failure. Tell your doctor if you have or have had kidney problems.
The protein content may increase, causing increased blood viscosity
Information on the original material ofGAMMAGARDS/D
GAMMAGARD S/D is manufactured from human plasma (the liquid part of the blood). When medicines are made from human blood or plasma, a number of measures are taken to prevent the possible transmission of infections to patients. These measures include careful selection of blood and plasma donors to ensure the exclusion of donors at risk of carrying infections and testing of each donation and plasma pool for potential viruses or infections. The manufacturers of these products also include a series of steps in the processing of the blood or plasma that can inactivate or eliminate viruses. Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of transmitting infections cannot be totally excluded. This applies to both known and emerging viruses and other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV), and for the non-enveloped hepatitis A virus (HAV). The measures taken may have limited value for non-enveloped viruses such as parvovirus B19.
Immunoglobulins have not been associated with hepatitis A or parvovirus B19 infections, possibly because the antibodies against these infections present in the product are protective.
It is recommended that each time you are given GAMMAGARD S/D, a record is kept of the name of the medicine and batch number administered to maintain a record of the batches used.
Using GAMMAGARDS/Dwith other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines or if you have been vaccinated in the last six weeks.
Infusion of immunoglobulins such as GAMMAGARD S/D may alter the effectiveness of some live virus vaccines such as measles, rubella, mumps and chickenpox. Therefore, after administration of these medicines, you may need to wait up to 3 months before receiving a live attenuated virus vaccine. You may need to wait up to 1 year after receiving immunoglobulins before receiving the measles vaccine.
Effects on blood tests
GAMMAGARD S/D contains a wide range of different antibodies, some of which may interfere with blood tests. If you have a blood test, please inform the analyst or your doctor that you have been given GAMMAGARD S/D.
Administration of Gammagard S/D may result in false positive readings in tests that rely on the detection of beta-D-glucans for the diagnosis of fungal infections; this may persist for several weeks after infusion of the product.
Pregnancy,breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
- No clinical trials have been conducted with GAMMAGARD S/D in pregnant or breast-feeding women. Years of clinical experience with antibody-containing medicines have shown that they are not expected to have harmful effects during pregnancy or on the baby.
- If you are breast-feeding, the antibodies in GAMMAGARD S/D may be present in breast milk. Therefore, your baby may be protected against certain infections.
- The effects of GAMMAGARD S/D on fertility have not been established.
Driving and using machines
Patients may experience reactions (e.g. dizziness or nausea) during treatment with GAMMAGARD S/D that could affect their ability to drive and use machines.
If this happens, wait until the reactions have disappeared.
Gammagard S/D 10 gcontainssodium and glucose
This medicine contains 668 mg of sodium (a major component of cooking/table salt) in each vial. This is equivalent to 34% of the maximum recommended daily intake of sodium for an adult.
This medicine contains glucose. Patients with diabetes mellitus should be aware that a 5% (50 mg/ml) solution of GAMMAGARD S/D contains 400 mg of glucose per gram of IgG. A 70 kg patient who receives a dose of 1 g/kg of IgG would receive 28 grams of glucose, which may affect their blood sugar levels.
3. How to use GAMMAGARD S/D
GAMMAGARD S/D is for intravenous administration (injection into a vein). It will be given to you by your doctor or nurse. The dose and frequency of infusion may vary depending on your situation and body weight.
At the start of the infusion, you will receive GAMMAGARD S/D at a low rate. Your doctor may gradually increase the infusion rate depending on whether you tolerate it well.
Use in children
In children (0 to 18 years), the same indications, dose and frequency of infusion are used as in adults.
If you use moreGAMMAGARDS/Dthan you should
If you receive more GAMMAGARD S/D than you should, the blood may become thicker (hyperviscosity). The thicker the blood, the harder it is to transport through the blood vessels in your body, so less oxygen will be carried to vital organs, such as the brain, lungs, etc. This can occur especially if you are a high-risk patient (e.g. an elderly patient or a patient with kidney or heart problems). Make sure to drink enough fluids to avoid dehydration and tell your doctor if you have any health problems.
In case of overdose or accidental administration, consult the Toxicology Information Service. Telephone 915 620 420.
4. Possible side effects
Like all medicines, GAMMAGARD S/D can cause side effects, although not everybody gets them. However, possible side effects can be reduced by decreasing the infusion rate.
The following side effects may occur after treatment with immunoglobulins (medicines like GAMMAGARD S/D):
- Frequent (may affect up to 1 in 10 people): chills, headache, fever, vomiting, nausea.
- Uncommon (may affect up to 1 in 100 people): mild lower back pain.
- Rare (may affect up to 1 in 1,000 people): cases of sudden drop in blood pressure, symptoms similar to eczema (transient skin reactions).
- Frequency not known (cannot be estimated from the available data): allergic reactions, including in patients who have not had reactions to previous infusions; temporary inflammation of the brain membranes (reversible aseptic meningitis); temporary reduction in red blood cell count; transient increases in liver function values (transaminases) and increased creatinine levels in the blood and acute kidney failure; formation of blood clots in the veins that have resulted in a heart attack, stroke, lung damage and deep vein thrombosis; joint pain; low blood pressure.
The following side effects have been reported by some patients with GAMMAGARD S/D in clinical trials and during post-marketing experience:
- Very common (may affect more than 1 in 10 people): none.
- Common (may affect up to 1 in 10 people): headache, flushing, nausea, vomiting, fatigue, chills, fever.
- Uncommon (may affect up to 1 in 100 people): flu, anxiety, agitation, abnormal drowsiness, blurred vision, feeling heartbeats, difficulty breathing, nosebleeds, diarrhea, upper abdominal pain, stomach upset, mouth inflammation, itching, skin rash, cold sweat, excessive sweating, back pain, muscle cramps, pain in arms and legs, chest pain, chest discomfort, abnormal sensation, feeling of cold, feeling of heat, flu-like symptoms, redness at the injection site, leakage of the medicine from the injection site, pain at the injection site, feeling of nausea/vomiting, pain, high blood pressure, changes in blood pressure.
- Frequency not known (cannot be estimated from the available data): non-infectious inflammation of the brain membranes; destruction of red blood cells; decrease in red blood cell count; decrease in platelet count; inflammation of the lymph nodes; allergic reactions of any severity, including anaphylactic shock; nervousness; dizziness; abnormal skin sensation; involuntary tremor; seizures; cerebral hemorrhage; transient stroke; migraine; loss of consciousness; light intolerance; visual disturbance; eye pain; central vein occlusion; heart attack; bluish discoloration of the skin; increased heart rate; decreased heart rate; low blood pressure; inflammation of the veins; blood clot formation; cough; throat tightness; decreased oxygen levels in the blood; hyperventilation; wheezing in the chest; bronchospasm; pulmonary embolism; fluid in the lungs; digestive disorders; abdominal pain; liver inflammation (non-transmissible); skin redness; skin rash; skin inflammation; allergic inflammation of the deep skin layers; muscle and joint pain; kidney failure; generalized weakness; swelling of body tissues; reactions at the injection site and infusion; positive Coombs test result.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of GAMMAGARD S/D
- Keep out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of that month.
- Do not use if particles or discoloration are observed.
- Do not store above 25°C.
- Do not freeze.
Keep the container in the outer packaging to protect it from light.
6. Container Contents and Additional Information
Composition ofGAMMAGARD S/D
The active ingredient of GAMMAGARD S/D is normal human immunoglobulin.
GAMMAGARD S/D can be reconstituted with sterile water for injectable preparations as a 5% protein solution (50 mg/ml) or 10% (100 mg/ml). At least 90% is immunoglobulin G (IgG).
The other components are human albumin, glycine, sodium chloride, and glucose monohydrate.
Appearance of the Product and Container Contents
GAMMAGARD S/D is a white or slightly yellowish lyophilized powder, substantially free of visible foreign particles. GAMMAGARD S/D is available in 5 g and 10 g containers.
Each container contains
- a 10 g powder vial
- 192 ml of water for injectable preparations
- a sterile transfer device
- a sterile administration kit with filter
Marketing Authorization Holder and Manufacturer:
Marketing Authorization Holder:
Baxalta Innovations GmbH
Industriestrasse 67
1221 Vienna
Austria
Manufacturer:
Baxalta Belgium Manufacturing SA
Boulevard René Branquart, 80 (Lessines)
B-7860-Belgium
Local Representative:
Takeda Farmacéutica España S.A.
Calle Albacete, 5, 9th floor,
Edificio Los Cubos
28027 Madrid
Spain
Tel: +34 91 790 42 22
This prospectus was approved in January 2021.
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
---------------------------------------------------------------------------------------------------------------------------
THIS INFORMATION IS INTENDED ONLY FOR MEDICAL PROFESSIONALS OR HEALTHCARE PROFESSIONALS
Special Precautions for Storage
A chemical and physical stability of reconstituted GAMMAGARD S/D has been demonstrated for 24 hours at room temperature. From a microbiological point of view, the product should be used immediately, and the time and storage conditions before use are the responsibility of the user and should not normally exceed 24 hours at 2-8°C, provided that the reconstitution has been carried out under controlled and validated aseptic conditions.
Reconstitution: Use an aseptic technique:
After reconstitution, only clear or slightly opalescent and colorless or slightly yellowish solutions will be administered.
Bring the powder vial and the vial of water for injectable preparations (solvent) to room temperature. Maintain this temperature until the dissolution is complete.
- Solution at 5%
- Remove the protectors from the vials and clean the stoppers with a germicidal solution.
- Remove the protector covering the spike of the transfer device. Do not touch the spike.
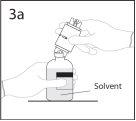 3a. Place the solvent vial on a smooth surface.
3a. Place the solvent vial on a smooth surface.
Use the exposed end of the spike to pierce the solvent vial through the center of the stopper.
PRECAUTION: if the spike is not inserted into the center of the stopper, it may become dislodged and the vacuum may be lost.
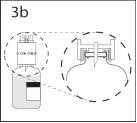 3b. Ensure that the vial neck is fully engaged in the device by pressing the transfer device firmly.
3b. Ensure that the vial neck is fully engaged in the device by pressing the transfer device firmly.
Remove the protector covering the other end of the spike while holding the transfer device. Do not touch the spike.
- Maintain the solvent vial with the transfer device connected at an angle with respect to the powder vial to prevent the solvent from spilling.
Note: do not place the solvent vial upside down as the solvent may spill.
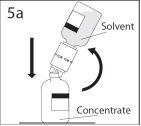 5a. Pierce the powder vial through the center of the stopper while quickly inverting the solvent vial to avoid spilling the solvent.
5a. Pierce the powder vial through the center of the stopper while quickly inverting the solvent vial to avoid spilling the solvent.
PRECAUTION: if the spike is not inserted into the center of the stopper, it may become dislodged and the vacuum may be lost.
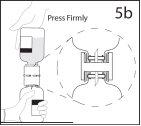 5b. Ensure that the vial neck is fully engaged in the device by pressing the solvent vial firmly.
5b. Ensure that the vial neck is fully engaged in the device by pressing the solvent vial firmly.
- After all the solvent has passed into the powder vial, remove the transfer device and the empty solvent vial. Immediately rotate the concentrate vial to mix the contents thoroughly.
PRECAUTION: do not shake. Avoid foam formation.
After a single use, discard the transfer device.
- Solution at 10%
- Remove the protectors from the vials and clean the stoppers with a germicidal solution.
- To prepare a 10% solution, it is necessary to withdraw half of the solvent volume. Table 2 describes the solvent volume to be withdrawn from each vial to achieve a 10% solution before connecting the transfer device. Using an aseptic technique, withdraw the unnecessary solvent volume using a sterile hypodermic syringe and needle. Discard the syringe and needle containing the unused solvent.
- Using the residual solvent in the solvent vial, follow steps 2-6 described in section A.
TABLE 2
10 g
Vial concentration
5% For reconstitution at 5%, do not withdraw any solvent
10% 96 ml
Administration. Use an aseptic technique
Follow the instructions for use of the administration kit included in the container. If another administration kit is used, ensure that it contains a similar filter.
Instructions for Use and Handling
The product should be brought to room temperature or body temperature before use.
Complete dissolution should be achieved within 30 minutes.
The resulting solution should be clear or slightly opalescent and colorless or slightly yellowish. Do not use solutions that are turbid or contain sediment. The reconstituted product should be visually inspected before administration to verify the absence of particles and coloration.
Disposal of unused medication and all materials that have come into contact with it should be carried out in accordance with local regulations.
Discard the transfer device after a single use.
Route of Administration
Intravenous.
If possible, it is recommended that the 10% solution of GAMMAGARD S/D be administered through the antecubital veins. This may reduce the likelihood of discomfort at the infusion site.
GAMMAGARD S/D at 5% (50 mg/ml) should be administered intravenously at an initial rate of 0.5 ml/kg/h. In general, it is recommended that patients who receive GAMMAGARD S/D for the first time or switch from another intravenous immunoglobulin to GAMMAGARD S/D start treatment with the lowest infusion rate and then increase to the maximum rate if they have previously tolerated several infusions at intermediate infusion rates.
If well tolerated, the infusion rate of the 5% solution can be gradually increased up to a maximum of 4 ml/kg/h. When switching from a 5% solution to a 10% solution, the infusion rate of the 10% solution should be initially low to maintain a comparable infusion rate of IgG protein. In many patients, it is possible to gradually increase the infusion rate of the 10% solution up to 8 ml/kg/h. The infusion rate will be adjusted individually according to the patient's tolerability.
Special Precautions
Any infusion-related adverse effect should be treated by reducing the rate or stopping the infusion.
Each time GAMMAGARD S/D is administered, it is recommended to indicate the product name and batch number.
Incompatibilities
GAMMAGARD S/D should not be mixed with other medications. It is recommended to administer GAMMAGARD S/D separately from other medications that the patient is receiving.
Recommended Dosage
INDICATION | DOSE | INJECTION/INFUSION FREQUENCY |
Replacement therapy in primary immunodeficiency Replacement therapy in secondary immunodeficiency Congenital AIDS Hypogammaglobulinemia (<4 g l) in patients who have received an allogeneic hematopoietic stem cell transplant< p> | Initial dose: 0.4 – 0.8 g/kg Continuation: 0.2-0.8 g/kg 0.2-0.4 g/kg 0.2-0.4 g/kg 0.2-0.4 g/kg | every 3-4 weeks to achieve a trough IgG level of at least 5-6 g/l. every 3-4 weeks to achieve a trough IgG level of at least 5-6 g/l. every 3-4 weeks every 3-4 weeks to achieve a trough IgG level above 5 g/l |
Immunomodulation: Primary immune thrombocytopenia Guillain-Barré syndrome Kawasaki disease | 0.8-1 g/kg or 0.4 g/kg /day 0.4 g/kg /day 1.6-2 g/kg or 2 g/kg | on the 1st day, which may be repeated once within the following three days for 2-5 days for 5 days in several doses over 2-5 days, together with acetylsalicylic acid in a single dose, together with acetylsalicylic acid. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GAMMAGARD S/D 10 G, POWDER AND SOLVENT FOR PERFUSION SOLUTIONDosage form: INJECTABLE PERFUSION, 100 mg/mlActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Instituto Grifols S.A.Prescription requiredDosage form: INJECTABLE PERFUSION, 100 mg/mlActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Instituto Grifols S.A.Prescription requiredDosage form: INJECTABLE PERFUSION, 100 mg/mlActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Instituto Grifols S.A.Prescription required
Online doctors for GAMMAGARD S/D 10 G, POWDER AND SOLVENT FOR PERFUSION SOLUTION
Discuss questions about GAMMAGARD S/D 10 G, POWDER AND SOLVENT FOR PERFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















