
GAMAVAN 360 micrograms/ml ORAL SOLUTION

How to use GAMAVAN 360 micrograms/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Gamavan 360 micrograms/ml oral solution is and what it is used for
- What you need to know before you take Gamavan 360 micrograms/ml oral solution
- How to take Gamavan 360 micrograms/ml oral solution
- Possible side effects
- Storage of Gamavan 360 micrograms/ml oral solution
- Contents of the pack and other information
Introduction
Package Leaflet: Information for the Patient
Gamavan 360micrograms/ml oral solution
(desmopressin)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you or your child get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Gamavan 360 micrograms/ml oral solution is and what it is used for
- What you need to know before you take Gamavan 360 micrograms/ml oral solution
- How to take Gamavan 360 micrograms/ml oral solution
- Possible side effects
- Storage of Gamavan 360 micrograms/ml oral solution
- Contents of the pack and other information
1. What Gamavan 360 micrograms/ml oral solution is and what it is used for
The active substance of this medicine is called desmopressin. Desmopressin is very similar to a substance produced naturally in the body (the hypophysial vasopressin hormone), which temporarily reduces the amount of urine produced by the body. This medicine is intended for oral use only.
Desmopressin is used to treat:
- Central diabetes insipidus, a disease characterized by extreme thirst and continuous production of large volumes of very diluted urine due to insufficient production of the vasopressin hormone.
- Primary nocturnal enuresis (urinary incontinence at night) in patients over 5 years of age with normal urine concentration capacity.
2. What you need to know before you take Gamavan 360 micrograms/ml oral solution
Do not take Gamavan:
- if you are allergic(hypersensitive) to desmopressinor to any of the other componentsof this medicine (listed in section 6),
- if you drinkexceptionally large amountsof liquid (have habitual or psychogenic polydipsia),
- if you take medicines that increase urine production(diuretics),
- if you have heart problems,
- if you have a predisposition to or suffer from sodium deficiencyin the blood (hyponatremia), inability to reduce fluid intake, for example, in memory disorders,
- if you have renal failure,
- if you have the “inappropriate antidiuretic hormone secretion syndrome” (SIADH).
Warnings and precautions
Consult your doctor or pharmacist before starting to take Gamavan
When taking desmopressin, avoid excessive fluid intake as it may cause fluid retention in the body and/or a decrease in sodium levels in the blood, with or without side effects (see section 4. Possible side effects).
Special care should be taken with desmopressin to avoid fluid retention in the body and decreased sodium levels in the blood in the following cases:
- If you are an elderly person
- If you have any medical condition that causes a water and/or electrolyte imbalancein the body, such as infection, fever, or stomach upset If you have a severe bladder problem or urinary evacuation disorder
- If you have asthma, epilepsy, and migraines
- If you have renal failure and/or cardiovascular disease. In the case of chronic renal failure, the antidiuretic effect of Gamavan would be lower than usual.
Other medicines and Gamavan
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, especially:
- tricyclic antidepressants or ISRS(used to treat depression),
- carbamazepine(used to treat epilepsy),
- chlorpromazine(used to treat psychosis or schizophrenia),
- pain and/or inflammation medicinescalled non-steroidal anti-inflammatory drugs (NSAIDs), such as indomethacin, ibuprofen, or acetylsalicylic acid,
- loperamide(used to treat diarrhea),
- diuretics.
These medicines increase the risk of fluid retention, which dilutes salt in the body.
- Dimethicone (used in the treatment of intestinal gas), as it decreases the absorption of desmopressin.
Taking Gamavan with food and drinks
See section 3 “How to take”.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
It is recommended to avoid taking Gamavan during pregnancy.
Desmopressin passes into breast milk. If you are going to be treated with desmopressin, you should stop breastfeeding.
Driving and using machines
Gamavan does not affect the ability to drive or use machines.
Important information about some of the components of Gamavan:
This medicine may cause allergic reactions (possibly delayed) because it contains methyl-parahydroxybenzoate sodium and propyl-parahydroxybenzoate sodium.
This medicine contains less than 23 mg of sodium (1mmol) per ml; that is, it is essentially “sodium-free”.
3. How to take Gamavan 360 micrograms/ml oral solution
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
At low doses, Gamavan may be affected by food intake. If you notice that Gamavan is less effective, you should take the medicine without food before increasing the dose.
When taking this medicine for nocturnal urinary incontinence, minimize fluid intakefrom 1hour beforetaking the Gamavan dose and up to 8hours after.
Treatment of central diabetes insipidus:
Adults and children:Your doctor will prescribe the most convenient dose for your case. The initial doserecommended is 0.25 ml (90 micrograms) three times a day. Subsequently, the doctor will adjust the dose according to each patient's response. The recommended daily doseis between 0.5 ml (180 micrograms) and 3 ml (1080 micrograms) of Gamavan. The maintenance dosevaries between 0.25 and 0.5 ml (90 – 180 micrograms) of Gamavan three times a day.
It is essential to note if symptoms of fluid retention in the body and/or decreased sodium levels in the blood appear (see section 4: Possible side effects), in which case treatment should be discontinued to change the dose.
Primary nocturnal enuresis (nocturnal urinary incontinence) in patients over 5 years of age:
Adults and children:The recommended initial dose is 0.5 ml (180 micrograms) of Gamavan one hour before bedtime. The dose may be increased to 1 ml (360 micrograms) of Gamavan if the initial dose is not sufficient. The need to continue treatment is usually checked every three months by introducing a treatment-free period of at least one week.
Instructions for use:
- Open the bottle (the seal is broken on the first opening).

- Insert the oral syringe into the dispenser and turn the bottle over to fill it with the dose to be administered.
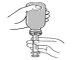
- Remove the oral syringe from the bottle and check that the bottom of the syringe shows the correct amount.
- Put the syringe in the mouth and administer the dose.
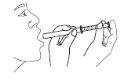
- Rinse with water after each use and close the bottle. Keep the bottle in the outer packaging to protect it from light.
If you take more Gamavan than you should
If you take more Gamavanthan you have been prescribed, contact your doctor or pharmacist immediately.
An overdose may prolong the effect of desmopressin and increase the risk of fluid accumulation in the body or decreased sodium levels in the blood. Symptoms may include headache, nausea, and vomiting, weight gain, and, in severe cases, convulsions. It is recommended to discontinue treatment, restrict fluid intake, and administer symptomatic treatment if necessary.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to take Gamavan
Do not take a double dose to make up for forgotten doses.
If you stop taking Gamavan
Do not stop taking Gamavan before finishing the treatment, as it may not have the expected effect. You should only change or discontinue treatment if your doctor indicates so.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following symptoms, stop taking Gamavan and consult a doctor or go to the hospital immediately:
Frequency not known(cannot be estimated from the available data)
- symptoms of fluid retentionin the body, such as severe or prolonged headache, dizziness, feeling or being sick, weight gain, and, in severe cases, convulsions or loss of consciousness (hyponatremia)
- allergic reactionssuch as rash, itching, fever, swelling of the lips, face, throat, or tongue that causes difficulty swallowing or breathing
- symptoms of sodium accumulationin the body, such as confusion, muscle contractions, or spasms (hypernatremia)
Tell your doctor or pharmacistif you notice any of these other side effects:
Common (may affect up to 1 in 10 people)
- abdominal pain, nausea, high blood pressure, diarrhea, constipation, bladder and kidney problems, swelling,
- headache
Uncommon(may affect up to 1 in 100 people)
- sudden and exaggerated mood changes, aggression, bladder and kidney problems, swelling of hands and feet, fatigue
Rare (may affect up to 1 in 1,000 people)
- anxiety, nightmares, mood changes, drowsiness, high blood pressure, irritability
Frequency not known (cannot be estimated from the available data)
- loss of consciousness, weakness, convulsions, emotional disorders, abnormal behavior, depression, hallucinations (seeing, hearing, or feeling things that are not present), insomnia, attention disorders, restlessness, nosebleeds, sweating, skin rashes, hives, skin allergies.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible that they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Gamavan 360 micrograms/ml oral solution
Keep this medicine out of the sight and reach of children.
This medicine does not require special storage conditions.
Keep in the original packaging.
After the first opening, store below 25 °C for a maximum of 8 weeks.
Do not use this medicine after the expiry date stated on the packaging or label after CAD or EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and other information
Gamavan composition
- The active substance is desmopressin. Each ml of the oral solution contains 360 micrograms of desmopressin (as acetate).
- The other components are:
- methyl-parahydroxybenzoate sodium (E-219)
- propyl parahydroxybenzoate sodium (E-217)
- hydrochloric acid (for pH adjustment)
- purified water
Appearance of the product and pack contents
Gamavanis a transparent solution included in an amber glass bottle with a plastic dispenser, provided with a screw cap also made of plastic. The bottle contains 15 ml of solution. The bottle is packaged in a cardboard box with a 1.5 ml dosing syringe. The syringe is graduated from 0 to 1.5 ml with divisions every 0.1 ml. The graduations corresponding to the doses of 0.25 ml, 0.5 ml, and 1.0 ml are specifically marked.
Marketing authorization holder
GP-Pharm, S.A.
Polígono Industrial Els Vinyets – Els Fogars, sector 2
Carretera Comarcal C244, Km 22,
08777 – Sant Quintí de Mediona (Barcelona) SPAIN
Manufacturer
Laboratorio Reig Jofré S.A.,
Gran Capitàn nº 10,
08970 Sant Joan Despí – Barcelona
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
France | NIWINUR 360 micrograms/ml, oral solution |
Spain | Gamavan 360 micrograms/ml oral solution |
Date of the last revision of this leaflet: May 2021
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GAMAVAN 360 micrograms/ml ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 360mcg/ml desmopressin (anhydrous base)Active substance: desmopressinManufacturer: Laboratorio Reig Jofre, S.A.Prescription requiredDosage form: SUBLINGUAL TABLET, 120 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: SUBLINGUAL TABLET, 240 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription required
Online doctors for GAMAVAN 360 micrograms/ml ORAL SOLUTION
Discuss questions about GAMAVAN 360 micrograms/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















