GALAFOLD 123 mg HARD CAPSULES

How to use GALAFOLD 123 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Galafold 123 mg Hard Capsules
Migalastat
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Galafold and what is it used for
- What you need to know before you take Galafold
- How to take Galafold
- Possible side effects
- Storage of Galafold
- Contents of the pack and other information
1. What is Galafold and what is it used for
Galafold contains the active substance migalastat.
This medicine is used for the long-term treatment of Fabry disease in adults and adolescents aged 12 years and older with certain genetic mutations (changes in genetic material).
Fabry disease is caused by a deficiency of an enzyme called alpha-galactosidase A (α-Gal A). Depending on the type of mutation (change in genetic material) of the gene that produces α-Gal A, the enzyme may not work properly or be completely absent. This enzyme deficiency leads to abnormal accumulations of a fatty substance called globotriaosylceramide (GL-3) in the kidneys, heart, and other organs, causing the symptoms of Fabry disease.
This medicine works by stabilizing the enzyme that the body produces naturally, so that it can work better to reduce the amount of GL-3 accumulated in cells and tissues.
2. What you need to know before you take Galafold
Do not take Galafold:
- if you are allergic to migalastat or any of the other ingredients of this medicine (listed in section 6).
Warnings and Precautions
The 123 mg migalastat capsules are not indicated for children (≥12 years) weighing less than 45 kg.
Tell your doctor before taking Galafold if you are using enzyme replacement therapy.
Do not take Galafold if you are also using enzyme replacement therapy.
While taking Galafold, your doctor should assess your condition and whether your medicine is working every 6 months. If your condition worsens, your doctor should perform additional tests or stop treatment with Galafold.
Consult your doctor before taking Galafold if your kidney function is severely impaired, as Galafold is not recommended for use in patients with severe renal impairment (with an eGFR below 30 ml/min/1.73 m2).
Children
Children <12 years
This medicine has not been studied in children under 12 years of age. Therefore, the safety and efficacy in this age group have not been established.
Other medicines and Galafold
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines. This includes medicines obtained without a prescription, such as supplements or herbal medicines.
Tell your doctor especially if you take medicines or supplements that contain caffeine, as these medicines may affect the activity of Galafold if taken during the fasting period.
Be aware of the medicines you take. Keep a list of them and show it to your doctor and pharmacist each time you get a new medicine.
Pregnancy, breastfeeding, and fertility
Pregnancy
There are very limited data on the use of this medicine in pregnant women. Galafold is not recommended during pregnancy. If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Women who can become pregnant must use an effective method of contraception while using Galafold.
Breastfeeding
Do not take this medicine if you are breastfeeding until you have talked to your doctor, pharmacist, or nurse. It is not known if this medicine is excreted in breast milk. Your doctor will decide whether you should stop breastfeeding or stop treatment with Galafold, considering the benefit of breastfeeding for the baby and the benefit of Galafold for the mother.
Male fertility
It is not known if this medicine affects male fertility. The effects of Galafold on fertility in humans have not been studied.
Female fertility
It is not known if this medicine affects female fertility.
If you are planning to become pregnant, consult your doctor, pharmacist, or nurse.
Driving and using machines
This medicine is not expected to affect your ability to drive or use machines.
3. How to take Galafold
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor, pharmacist, or nurse.
The recommended dose is one capsule every two days at the same time of day. Do not take Galafold on two consecutive days.
Do not eat any food or caffeine for at least 2 hours before and 2 hours after taking the medicine. This 4-hour fasting period around the time of taking the medicine is necessary to allow the medicine to be fully absorbed.
During the 4-hour fasting period, you can consume water (natural, flavored, sugared), fruit juices without pulp, and non-caffeinated carbonated drinks.
Swallow the capsules whole. Do not split, crush, or chew the capsules.
Figure A
| Step 1: Remove the adhesive strip that keeps the flap closed. Lift the flap to open the Galafold carton (see Figure A). |
Figure B: Open carton
| Step 2: Press the purple tab with your thumb on the leftside of the carton without releasing it (see Figure B) and proceed to step 3. |
Figure C
| Step 3: Now GRASP the tab on the rightside, where it says “PULL HERE”, and pull out the folded blister pack (see Figure C). |
Figure D: Front of the blister pack
| Step 4: Unfold the blister pack (see Figure D). |
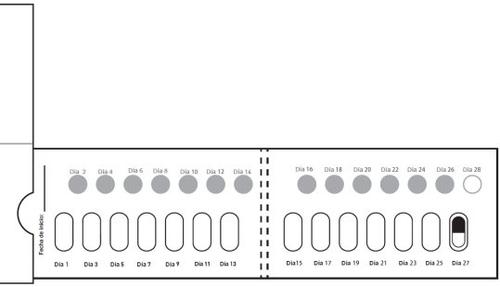
How to take the Galafold capsules: One Galafold blister pack = 14 hard capsules = 28 days of treatment with Galafold and 14 white circles. The white circles are to remind you to take Galafold every twodays. The arrow indicates to the patient that they should start the next 2 weeks of treatment. Figure E: Front of the blister pack
| |
Figure F: Front of the blister pack
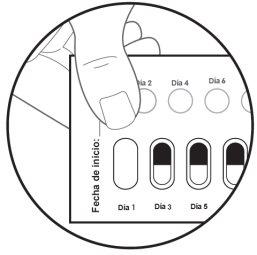
| Step 5: On the first day you start with this medicine, mark the date on the new blister pack (see Figure F). |
Figure G: Back of the blister pack
| Step 6: TURN OVER the blister pack to see the back. LOCATE the capsule you are going to remove. FOLD the blister pack as indicated here (see Figure G). Note: When folding the blister pack, the perforated oval stands out. |
| Step 7: REMOVE the perforated oval (see Figure H). Note: When removing the oval, the white backing sheet may still remain. This is correct. |
Figure I: Front of the blister pack
| Step 8: TURN OVER the blister pack to see the front. PUSH the capsule to remove it (see Figure I). |
Figure J: Front of the blister pack
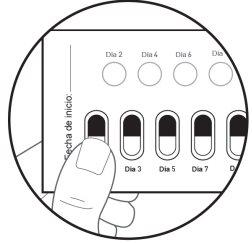
| Step 9: The next day, move to the white circle in the top row marked as Day 2. Press the white circle to perforate it (see Figure J). Note: Perforating this white circle will help you remember which days you should not take the medicine. Take 1 Galafold capsule every twodays. Close and store the box after each use. |
After Day 2, move to Day 3 of the blister pack. Alternate the days you take the capsule and perforate the white circles, until day 28, including this last day. Figure K: Front of the unfolded blister pack
|
If you take more Galafold than you should
If you take more capsules than you should, stop taking the medicine and consult your doctor. You may experience headache and dizziness.
If you forget to take Galafold
If you forget to take the capsule at the usual time and remember later, you can take the capsule only if it is within the next 12 hours after the usual time you take the dose. If more than 12 hours have passed, you should take Galafold the next day at the usual time, according to the every-other-day dosing schedule. Do not take a double dose to make up for forgotten doses.
If you stop taking Galafold
Do not stop treatment without talking to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common: may affect more than 1 in 10 people
- Headache
Common: may affect up to 1 in 10 people
|
|
|
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Galafold
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister pack after EXP. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions. Store in the original package to protect from moisture.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Galafold
- The active ingredient is migalastat. Each capsule contains migalastat hydrochloride equivalent to 123 mg of migalastat
- The other ingredients are:
Capsule content: pregelatinized starch (corn) and magnesium stearate
Capsule shell: gelatin, titanium dioxide (E171), and indigo carmine (E132)
Printing ink: shellac, iron oxide black, and potassium hydroxide
Appearance of Galafold and Package Contents
Hard blue and white opaque capsules marked with «A1001» in black ink, hard capsule size 2 (6.4 x 18.0 mm) containing a white to light brown powder.
Galafold is available in a blister pack of 14 capsules.
Marketing Authorization Holder
Amicus Therapeutics Europe Limited
Block 1, Blanchardstown Corporate Park
Ballycoolin Road
Blanchardstown, Dublin
D15 AKK1
Ireland
Tel: +353 (0) 1 588 0836
Fax: +353 (0) 1 588 6851
e-mail: [email protected]
Manufacturer
Almac Pharma Services (Ireland) Limited
Finnabair Industrial Estate
Dundalk, Co. Louth
A91 P9KD
Ireland
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder (if you cannot contact your Amicus representative by phone, please contact us through the email address listed below):
Belgium Amicus Therapeutics Europe Limited Tel: (+32) 0800 89172 e-mail: [email protected] | Lithuania Amicus Therapeutics Europe Limited Tel.: (+370) 8800 33167 e-mail: [email protected] |
Bulgaria Amicus Therapeutics Europe Limited Tel: (+359) 00800 111 3214 e-mail: [email protected] | Luxembourg Amicus Therapeutics Europe Limited Tel: (+352) 800 27003 e-mail: [email protected] |
Czech Republic Amicus Therapeutics Europe Limited Tel.: (+420) 800 142 207 e-mail: [email protected] | Hungary Amicus Therapeutics Europe Limited Tel.: (+36) 06 800 21202 e-mail: [email protected] |
Denmark Amicus Therapeutics Europe Limited Tel: (+45) 80 253 262 e-mail: [email protected] | Malta Amicus Therapeutics Europe Limited Tel: (+356) 800 62674 e-mail: [email protected] |
Germany Amicus Therapeutics GmbH Tel.: (+49) 0800 000 2038 e-mail: [email protected] | Netherlands Amicus Therapeutics BV Tel: (+31) 0800 022 8399 e-mail: [email protected] |
Estonia Amicus Therapeutics Europe Limited Tel: (+372) 800 0111 911 e-mail: [email protected] | Norway Amicus Therapeutics Europe Limited Tel: (+47) 800 13837 e-mail: [email protected] |
Greece Amicus Therapeutics Europe Limited Tel.: (+30) 00800 126 169 e-mail: [email protected] | Austria Amicus Therapeutics Europe Limited Tel: (+43) 0800 909 639 e-mail: [email protected] |
Spain Amicus Therapeutics S.L.U. Tel: (+34) 900 941 616 e-mail: [email protected] | Poland Amicus Therapeutics Europe Limited Tel.: (+48) 0080 012 15475 e-mail: [email protected] |
France Amicus Therapeutics SAS Tel: (+33) 0 800 906 788 e-mail: [email protected] | Portugal Amicus Therapeutics Europe Limited Tel: (+351) 800 812 531 e-mail: [email protected] |
Croatia Amicus Therapeutics Europe Limited Tel: (+385) 0800 222 452 e-mail: [email protected] | Romania Amicus Therapeutics Europe Limited Tel.: (+40) 0808 034 288 e-mail: [email protected] |
Ireland Amicus Therapeutics Europe Limited Tel: (+353) 1800 936 230 e-mail: [email protected] | Slovenia Amicus Therapeutics Europe Limited Tel.: (+386) 0800 81794 e-mail: [email protected] |
Iceland Amicus Therapeutics Europe Limited Tel: (+354) 800 7634 e-mail: [email protected] | Slovakia Amicus Therapeutics Europe Limited Tel.: (+421) 0800 002 437 e-mail: [email protected] |
Italy Amicus Therapeutics S.r.l. Tel: (+39) 800 795 572 e-mail: [email protected] | Finland Amicus Therapeutics Europe Limited Tel: (+358) 0800 917 780 e-mail: [email protected] |
Cyprus Amicus Therapeutics Europe Limited Tel.: (+357) 800 97595 e-mail: [email protected] | Sweden Amicus Therapeutics Europe Limited Tel: (+46) 020 795 493 e-mail: [email protected] |
Latvia Amicus Therapeutics Europe Limited Tel.: (+371) 800 05391 e-mail: [email protected] | United Kingdom (Northern Ireland) Amicus Therapeutics, UK Limited Tel: (+44) 08 0823 46864 e-mail: [email protected] |
Date of Last Revision of this Leaflet
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GALAFOLD 123 mg HARD CAPSULESDosage form: TABLET, UnknownActive substance: sodium phenylbutyrateManufacturer: Immedica Pharma AbPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, UnknownActive substance: sodium phenylbutyrateManufacturer: Immedica Pharma AbPrescription requiredDosage form: CAPSULE, 84 mg of eliglustat (as tartrate)Active substance: eliglustatManufacturer: Sanofi B.V.Prescription required
Online doctors for GALAFOLD 123 mg HARD CAPSULES
Discuss questions about GALAFOLD 123 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




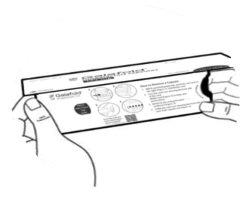
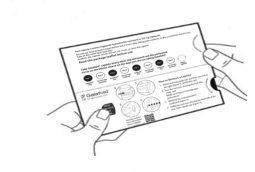

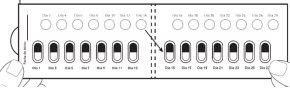
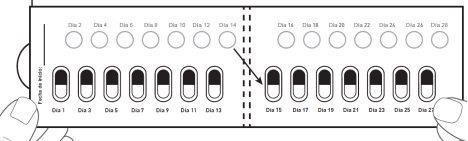
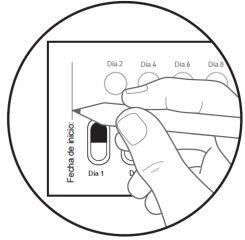
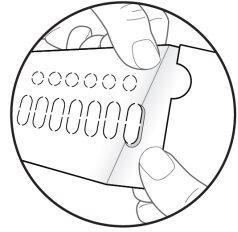
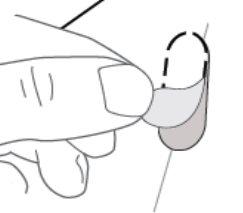 Figure H: Back of the blister pack
Figure H: Back of the blister pack