
CERDELGA 84 mg HARD CAPSULES

How to use CERDELGA 84 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Cerdelga 21mg Hard Capsules
Cerdelga 84mg Hard Capsules
eliglustat
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Cerdelga and what is it used for
- What you need to know before you take Cerdelga
- How to take Cerdelga
- Possible side effects
- Storing Cerdelga
- Contents of the pack and other information
1. What is Cerdelga and what is it used for
Cerdelga contains the active substance eliglustat and is used for the long-term treatment of adults and children from 6 years of age who weigh at least 15 kg with type 1 Gaucher disease.
When used in children, Cerdelga is intended for those children whose disease is under control with enzyme replacement therapy. Your doctor will determine if Cerdelga is suitable for you or your child before you start taking it with a simple laboratory test.
Type 1 Gaucher disease is a rare inherited disorder in which the body does not break down a substance called glucosylceramide properly. As a result, glucosylceramide accumulates in the spleen, liver, and bones. This accumulation prevents these organs from functioning properly. Cerdelga contains the active substance eliglustat, which reduces the production of glucosylceramide and thus prevents its accumulation. In turn, this helps the affected organs to function better.
There are differences between people in the speed at which the body breaks down this medicine. Therefore, the amount of medicine in the blood may differ from one patient to another, which may affect how a patient responds to treatment. Cerdelga is intended for use in patients who break down the medicine at a normal speed (so-called intermediate and rapid metabolizers) or at a low speed (so-called slow metabolizers).
Type 1 Gaucher disease is a lifelong condition, so you will need to keep taking this medicine as directed by your doctor to get the most benefit from treatment.
2. What you need to know before you take Cerdelga
Do not take Cerdelga
- If you are allergic to eliglustat or any of the other ingredients of this medicine (listed in section 6).
- If you are an intermediate or rapid metabolizer and are using medicines called potent or moderate CYP2D6 inhibitors (such as quinidine and terbinafine) in combination with potent or moderate CYP3A inhibitors (such as erythromycin and itraconazole). The combination of these medicines will interfere with your body's ability to break down Cerdelga and may result in higher levels of the active substance in the blood (see section "Other medicines and Cerdelga" for an extended list of medicines).
- If you are a slow metabolizer and are using medicines known as potent CYP3A inhibitors (e.g. itraconazole). Medicines of this type will interfere with your body's ability to metabolize Cerdelga and this may result in higher levels of the active substance in your blood (see the section "Other medicines and Cerdelga" for an extended list of medicines).
- If you are a rapid metabolizer and have severely impaired liver function.
- If you are a rapid metabolizer and have mildly or moderately impaired liver function while taking a potent or moderate CYP2D6 inhibitor.
Warnings and precautions
Talk to your doctor or pharmacist before starting Cerdelga if:
- you are currently being treated with any of the medicines that appear in the section "Other medicines and Cerdelga" or are about to start treatment with them.
- you have had a heart attack or heart failure.
- you have a low heart rate.
- you have an irregular or abnormal heart rhythm, including a heart condition called long QT syndrome.
- you have other heart problems.
- you are taking an anti-arrhythmic medicine (used to treat an irregular heart rhythm) such as quinidine, amiodarone, or sotalol.
- you are a rapid metabolizer and have moderately impaired liver function.
- you are an intermediate or slow metabolizer and have impaired liver function at any level.
- you are an intermediate or slow metabolizer and have impaired kidney function.
- you are a patient with end-stage renal disease (ESRD).
Children and adolescents
Cerdelga is not intended for use in children under 6 years of age or weighing less than 15 kg.
Other medicines and Cerdelga
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Medicines that must not be taken together with Cerdelga
Cerdelga must not be used with certain types of medicines. These medicines can interfere with your body's ability to break down Cerdelga, which may result in higher levels of Cerdelga in the blood. These medicines are known as potent or moderate CYP2D6 inhibitors and potent or moderate CYP3A inhibitors. There are many medicines in these categories, and depending on how your body metabolizes Cerdelga, the effects may vary from person to person. Talk to your doctor about these medicines before starting Cerdelga. Your doctor will determine which medicines you can use based on how quickly your body metabolizes eliglustat.
Medicines that may increase the level of Cerdelga in the blood:
- paroxetine, fluoxetine, fluvoxamine, duloxetine, bupropion, moclobemide -antidepressants(used to treat depression)
- dronedarone, quinidine, verapamil -anti-arrhythmics(used to treat irregular heartbeat)
- ciprofloxacin, clarithromycin, erythromycin, telithromycin -antibiotics(used to treat infections)
- terbinafine, itraconazole, fluconazole, posaconazole, voriconazole -antifungals(used to treat fungal infections)
- mirabegron -used to treat overactive bladder
- cinacalcet -calcimimetic(used in some patients with dialysis and in certain cancers)
- atazanavir, darunavir, fosamprenavir, indinavir, lopinavir, ritonavir, saquinavir, tipranavir -antiretrovirals(used to treat HIV infection)
- cobicistat -used to enhance the effects of antiretrovirals (used to treat HIV)
- aprepitant -antiemetic(used to reduce vomiting)
- diltiazem -antihypertensive(used to increase blood flow and reduce heart rate)
- conivaptan -diuretic(used to raise low sodium levels in the blood)
- boceprevir, telaprevir -antivirals(used to treat hepatitis C)
- imatinib -anticancer(used to treat cancer)
- amlodipine, ranolazine -used to treat angina
- cilostazol -used to treat leg pain when walking due to poor blood circulation
- isoniazid -used to treat tuberculosis
- cimetidine, ranitidine -antacids(used to treat indigestion)
- goldenseal -(also known as Hydrastis canadensis), a herbal medicine available without prescription, used to aid digestion.
Medicines that may reduce the level of Cerdelga in the blood:
- rifampicin, rifabutin -antibiotics(used to treat infections)
- carbamazepine, phenobarbital, phenytoin -antiepileptics(used to treat epilepsy and seizures)
- St. John's Wort (also known as Hypericum perforatum) -a herbal medicine available without prescription, used to treat depressionand other disorders.
Cerdelga may increase the level of the following types of medicines in the blood:
- dabigatran -anticoagulant(used to thin the blood)
- phenytoin -antiepileptic(used to treat epilepsy and seizures)
- nortriptyline, amitriptyline, imipramine, desipramine -antidepressants(used to treat depression)
- phenothiazines -antipsychotics(used to treat schizophrenia and psychosis)
- digoxin -used to treat heart failure and atrial fibrillation
- colchicine -used to treat gout
- metoprolol -used to lower blood pressure and/or heart rate
- dextromethorphan -cough suppressant
- atomoxetine -used to treat attention deficit hyperactivity disorder (ADHD)
- pravastatin -used to lower cholesterol and prevent heart disease
Taking Cerdelga with food and drinks
Avoid consuming grapefruit or grapefruit juice, as it may increase the level of Cerdelga in the blood.
Pregnancy, breast-feeding, and fertility
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
It has been shown that the active substance of this medicine passes into breast milk in very small amounts in animals. Breast-feeding is not recommended during treatment with this medicine. If you are breast-feeding, inform your doctor.
There are no known effects on fertility at normal doses.
Driving and using machines
Cerdelga may affect your ability to drive or use machines in patients who experience dizziness after administration.
Cerdelga contains lactose
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
3. How to take Cerdelga
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
Cerdelga is available in 2 different doses. The hard capsules containing 84 mg of eliglustat are greenish-blue and white, and the hard capsules containing 21 mg of eliglustat are completely white. When giving this medicine to your child, make sure they are taking the correct dose.
Cerdelga should be taken orally in children who can swallow the capsule whole.
The hard capsules of Cerdelga should be taken whole with water at the same time every day. They can be taken with or without food. Patients taking the dose twice a day should take one dose in the morning and one dose in the evening.
Do not open, crush, dissolve, or chew the hard capsule before swallowing. If you cannot swallow the capsule whole, inform your doctor.
The mixture of the capsule contents (eliglustat powder) with food or drinks has not been studied.
Recommended dose for adults
If you are an intermediate or rapid metabolizer:
Swallow one 84 mg capsule whole twice a day with water. It can be taken with or without food. Take one capsule in the morning and one in the evening.
If you are a slow metabolizer:
Swallow one 84 mg capsule whole once a day with water. It can be taken with or without food. Take one capsule at the same time each day.
Recommended dose for children
The amount of this medicine your child takes depends on their body weight and how they metabolize the medicine. The doctor will determine this before starting treatment.
Weight | If your child is an intermediate or rapid metabolizer | If your child is a slow metabolizer |
50 kg or more | One 84 mg capsule (greenish-blue and white) twice a day | One 84 mg capsule (greenish-blue and white) once a day |
25 kg to less than 50 kg | One 84 mg capsule (greenish-blue and white) twice a day | Two 21 mg capsules (white) once a day |
15 kg to less than 25 kg | Two 21 mg capsules (white) twice a day | One 21 mg capsule (white) once a day |
Keep taking Cerdelga every day unless your doctor tells you to stop.
How to dispense the 21 mg hard capsule
Break the foil that covers the capsule with your thumb or index finger and push the capsule out.
How to remove the blister from the wallet for the 84 mg hard capsule
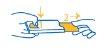
Pressing with your thumb and index finger together at one end of the wallet (1), gently pull the blister to open the wallet (2).
If you take more Cerdelga than you should
If you take more capsules than you were told, talk to your doctor immediately. You may experience dizziness with loss of balance, low heart rate, nausea, vomiting, and fainting.
If you forget to take Cerdelga
Take the next capsule at the usual time. Do not take a double dose to make up for forgotten doses.
If you stop taking Cerdelga
Do not stop taking Cerdelga without talking to your doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common(may affect up to 1 in 10 people):
- Headache
- Dizziness
- Change in taste (dysgeusia)
- Palpitations
- Sore throat
- Cough
- Heartburn (dyspepsia)
- Stomach pain (upper abdominal pain)
- Diarrhea
- Nausea
- Constipation
- Abdominal pain
- Acid reflux disease (gastroesophageal reflux disease)
- Bloating (abdominal distension)
- Stomach inflammation (gastritis)
- Difficulty swallowing (dysphagia)
- Vomiting
- Dry mouth
- Gas (flatulence)
- Dry skin
- Hives (urticaria)
- Joint pain (arthralgia)
- Pain in arms, legs, or back
- Fatigue (fatigue)
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Cerdelga
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, wallet, and blister after "EXP". The expiry date is the last day of the month shown.
No special storage conditions are required.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
Cerdelga composition
The active ingredient is eliglustat (as tartrate).
Cerdelga 21 mg hard capsules
Each hard capsule contains 21 mg of eliglustat.
The other ingredients are:
- In the capsule: microcrystalline cellulose (E460), lactose monohydrate (see section 2 "Cerdelga contains lactose"), hypromellose 15 mPa.S, 2910 and glycerol dibehenate.
- In the capsule shell: gelatin (E441), potassium and aluminum silicate (E555), titanium dioxide (E171).
- In the printing ink: shellac, black iron oxide (E172), propylene glycol (E1520) and concentrated ammonia solution (E527).
Cerdelga 84 mg hard capsules
Each hard capsule contains 84 mg of eliglustat.
The other ingredients are:
- In the capsule: microcrystalline cellulose (E460), lactose monohydrate (see section 2 "Cerdelga contains lactose"), hypromellose and glycerol dibehenate.
- In the capsule shell: gelatin (E441), potassium and aluminum silicate (E555), titanium dioxide (E171), yellow iron oxide (E172) and indigotine (E132).
- In the printing ink: shellac, black iron oxide (E172), propylene glycol (E1520) and concentrated ammonia solution (E527).
Appearance of Cerdelga and container contents
Cerdelga 21 mg hard capsules
The Cerdelga 21 mg hard capsules have an opaque white nacreous cap and an opaque white nacreous body with the inscription "GZ04" printed in black on the capsule.
Package sizes of 56 hard capsules in 4 blisters of 14 capsules each.
Cerdelga 84 mg hard capsules
The Cerdelga 84 mg hard capsules have an opaque blue-green nacreous cap and an opaque white nacreous body with the inscription "GZ02" printed in black on the capsule.
Package sizes of 14 hard capsules in 1 blister, 56 hard capsules in 4 blisters of 14 capsules each or 196 hard capsules in 14 blisters of 14 capsules each.
Only some package sizes may be marketed.
Marketing authorization holder
Sanofi B.V.
Paasheuvelweg 25 1105 BP Amsterdam
Netherlands
Manufacturer
Cerdelga 21 mg hard capsule
Patheon France
40 Boulevard de Champaret
Bourgoin Jallieu
38300
France
Cerdelga 84 mg hard capsule
Sanofi Winthrop Industrie
30-36 avenue Gustave Eiffel
37100 Tours
France
Sanofi Winthrop Industrie
1 rue de la Vierge
Ambares et Lagrave
33565 Carbon Blanc cedex
France
Genzyme Ireland Ltd
IDA Industrial Park
Old Kilmeaden Road
Waterford
Ireland
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien/ Luxembourg/Luxemburg Sanofi Belgium Tel: + 32 2 710 54 00 | Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Magyarország SANOFI-AVENTIS Zrt. Tel.: +36 1 505 0050 |
Ceskárepublika Sanofi s.r.o. Tel: +420 233 086 111 | Malta Sanofi S.r.l. Tel: +39 02 39394275 |
Danmark Sanofi A/S Tlf.: +45 45 16 70 00 | Nederland Sanofi B.V. Tel: +31 20 245 4000 |
Deutschland Sanofi-Aventis Deutschland GmbH Tel.: 0800 04 36 996 Tel. from abroad: +49 69 305 70 13 | Norge sanofi-aventis Norge AS Tlf: + 47 67 10 71 00 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Österreich sanofi-aventis GmbH Tel: + 43 1 80 185 - 0 |
Sanofi-Aventis Μονοπρ?σωπη AEBE +30 210 900 1600 | Polska Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Portugal Sanofi – Produtos Farmacêuticos, Lda. Tel: +351 21 35 89 400 |
France Sanofi Winthrop Industrie Tél: 0 800 222 555 Call from abroad: +33 1 57 63 23 23 | România Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tel: 800 536 389 | Suomi/Finland Sanofi Oy Puh/Tel: + 358 201 200 300 |
Κ?προς C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sverige Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CERDELGA 84 mg HARD CAPSULESDosage form: TABLET, UnknownActive substance: sodium phenylbutyrateManufacturer: Immedica Pharma AbPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, UnknownActive substance: sodium phenylbutyrateManufacturer: Immedica Pharma AbPrescription requiredDosage form: CAPSULE, 200 mgActive substance: trientineManufacturer: Univar Solutions B.V.Prescription required
Online doctors for CERDELGA 84 mg HARD CAPSULES
Discuss questions about CERDELGA 84 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















