
FLOGOPATCH 70 MG MEDICATED ADHESIVE DRESSING


How to use FLOGOPATCH 70 MG MEDICATED ADHESIVE DRESSING
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Flogopatch 70 mg Medicinal Adhesive Patch
Etofenamate
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
- You should consult a doctor if your condition worsens or does not improve after 7 days.
Contents of the Package Leaflet
- What Flogopatch is and what it is used for
- What you need to know before using Flogopatch
- How to use Flogopatch
- Possible side effects
- Storage of Flogopatch
- Package Contents and Additional Information
1. What Flogopatch is and what it is used for
Etofenamate, the active ingredient of this medication, belongs to the group of non-steroidal anti-inflammatory drugs (NSAIDs), which have analgesic and anti-inflammatory properties.
Flogopatch is indicated for the short-term symptomatic treatment of local pain in acute ankle sprains without complications in adults.
You should inform your doctor if you do not improve or worsen after 7 days.
2. What you need to know before using Flogopatch
Do not use Flogopatch:
- If you are allergic to etofenamate or any of the other components of this medication (listed in section 6);
- If you have an allergy to other non-steroidal anti-inflammatory drugs (NSAIDs, medications used to treat pain and inflammation, e.g., acetylsalicylic acid or ibuprofen) that manifests as asthma, difficulty breathing (bronchospasm), runny nose, swelling, or hives;
- If you have skin lesions (e.g., abrasions, cuts, burns, open wounds), skin infections, or skin inflammation, or skin affected by exudative dermatitis or eczema;
- If you are in the third trimester of pregnancy (see section "Pregnancy, Breastfeeding, and Fertility");
- In eyes, lips, or mucous membranes.
Warnings and Precautions
If a skin reaction occurs, remove the medicinal adhesive patch immediately and discontinue treatment.
In order to minimize the occurrence of side effects, it is recommended to use it for the shortest time necessary to control the symptoms.
Do not use a bandage to hold the medicinal adhesive patch in place.
Difficulty breathing (bronchospasm) may occur in patients who have previously suffered from bronchial asthma or allergies.
Avoid direct exposure to the sun or sunlamp radiation in the treated area during treatment and 2 weeks after removing the patch to reduce the risk of light sensitivity.
It cannot be ruled out that systemic side effects (side effects that affect entire organs, organ systems, or the whole body) may occur due to the application of the medicinal adhesive patch, if the medication is used on extensive areas of the skin (e.g., application of more than one patch - see section 3) and for a prolonged period.
Although it is expected that systemic effects will be minimal, the medicinal adhesive patch should be used with caution in patients with:
- Kidney, liver, or heart problems
- If you have or have had gastrointestinal ulcers, intestinal inflammation, or a tendency to bleed.
Non-steroidal anti-inflammatory drugs (NSAIDs) should be used with caution if you are an elderly person, as you are more likely to suffer from side effects.
Do not use other medications that contain etofenamate or other non-steroidal anti-inflammatory drugs (NSAIDs) at the same time, either topically (on the skin) or orally.
Children and Adolescents
Flogopatch should not be used in children and adolescents under 18 years of age, as the efficacy and safety of this medication have not been established in this age group.
Using Flogopatch with Other Medications
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
If Flogopatch is used correctly, only a small amount of etofenamate is absorbed into the body, so it is unlikely to interact with other medications.
Pregnancy, Breastfeeding, and Fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Do not use this medication during the third trimester of pregnancy.
Only use this medication during the first and second trimester of pregnancy under medical supervision.
A small amount of the etofenamate degradation product, flufenamic acid, is excreted in breast milk. However, if your doctor considers it necessary, it may be possible to use Flogopatch for a short period during breastfeeding. If used during breastfeeding, Flogopatch should not be applied to the breast of the nursing mother, nor to any extensive area of the skin or for a prolonged period.
Driving and Using Machines
The influence of Flogopatch on the ability to drive and use machines is negligible or virtually negligible.
3. How to Use Flogopatch
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
Adults and Elderly Patients:
Apply one (1) patch every 12 hours (a total of 2 patches per day), for no more than 7 days.
Only one patch should be used at a time.
Do not exceed the recommended dose.
Method of Administration
For cutaneous use.
Use the patch only on intact, non-infected skin.
How to Apply the Patch
The patch should be placed on dry, wrinkle-free skin. If the site where the patch is to be applied is sweaty or has a lot of hair, this may interfere with the adhesion of the patch.
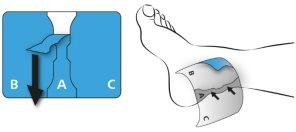
First, remove the protective film from the center of the patch (A) and press it directly onto the skin.
Do not touch the adhesive part of the patch with your fingers!
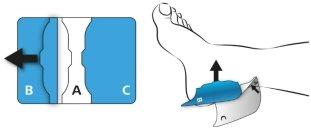
Hold the center of the patch (A) with the other hand and remove part (B) of the protective film by folding the patch outward and peeling off the protective film (B) at the protruding end (see the arrow). After removing the protective film, press the patch directly onto the skin.
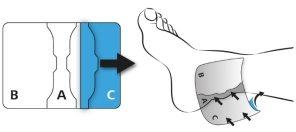
Remove part (C) of the protective film in the same way as part (B). Press the patch directly onto the skin.
Press the patch onto the skin for at least 30 seconds to ensure optimaladhesion.
After applying each patch, make sure to carefully close the envelope using the hermetic seal.
Duration of Treatment
For short-term use only
Flogopatch should be used for the shortest time necessary to control the symptoms.
The patch should not be used for more than 7 days. If there is no improvement after this period, or if symptoms worsen, please consult your doctor.
Do not exceed the recommended treatment duration.
If You Use More Flogopatch Than You Should
Seek medical attention immediately in case of accidental use by a child.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount used/ingested.
If You Forget to Use Flogopatch
Do not use a double dose to make up for the forgotten dose.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Discontinue use of this medication and contact your doctor immediately if you experience the following:
- Signs of allergic reaction such as asthma, unexplained wheezing or shortness of breath, itching, runny nose, or skin rashes
- Signs of hypersensitivity and skin reactions such as redness, swelling, peeling, blisters, scaling, or skin ulcers
You may experience the following side effects:
Uncommon (may affect up to 1 in 100 people)
Dermatitis, for example, skin redness, itching, burning, skin rash also with papule, pustule, or hives formation.
Rare (may affect up to 1 in 1,000 people)
Hypersensitivity reactions, local allergic reactions (contact dermatitis)
Very Rare (affects up to 1 in 10,000 people)
Skin swelling
Frequency Not Known (cannot be estimated from available data)
Light sensitivity (photosensitization)
The risk of side effects increases if the patch is used on an extensive area of the skin (e.g., application of more than one patch - see section 3) and for a prolonged period.
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Flogopatch
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date shown on the envelope and carton. The expiration date is the last day of the month indicated.
Do not store above 30°C
Used patches should be folded with the adhesive side inward and disposed of safely.
Medications should not be thrown down the drain or into the trash. Deposit the packaging and medications you no longer need at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Flogopatch
The active ingredient is etofenamate.
Each medicinal adhesive patch contains 70 mg of etofenamate.
The other components are:
Adhesive layer
Alpha-hydro-omega-hydroxypoly (dimethylsiloxane) polycondensed with dimethicone, macrogol 400, and refined olive oil.
Support
Bielastic polyester fabric.
Protective film
Fluoropolymer-coated polyester film.
Appearance of the Product and Package Contents
Each medicinal adhesive patch, 10 cm x 14 cm, consists of a white-colored fabric with a colorless and self-adhesive layer and a removable protective film.
Flogopatch is available in cartons containing a sealed envelope. Each envelope contains 2, 5, or 7 medicinal adhesive patches. The envelope is equipped with a hermetic seal to allow it to be closed after removing the patches.
Not all pack sizes may be marketed.
Marketing Authorization Holder
CHIESI ESPAÑA, S.A.U.
Plaça d’Europa, 41-43, 10th floor
08908 L’Hospitalet de Llobregat (Barcelona)
Spain
Manufacturer
mikle-pharm GmbH,
Sandgasse 17,
76829 Landau,
Germany
This medication is authorized in the Member States of the European Economic Area under the following names:
Austria/Germany: Lixim 70 mg wirkstoffhaltiges Pflaster
Belgium: Lixim 70 mg emplâtre médicamenteux / Lixim 70 mg wirkstoffhaltiges Pflaster / Lixim 70 mg pleister
Spain: Flogopatch 70 mg apósito adhesivo medicamentoso
Hungary: Lixim 70 mg gyógyszeres tapasz
Italy: Dorsiflex
Poland: Lixim
Portugal: Lixim 70 mg emplastro medicamentoso
Date of Last Revision of this Package Leaflet:June 2019
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLOGOPATCH 70 MG MEDICATED ADHESIVE DRESSINGDosage form: GEL, 100gActive substance: etofenamateManufacturer: Chiesi España S.A.U.Prescription not requiredDosage form: TOPICAL SOLUTION, 5000 mgActive substance: etofenamateManufacturer: Chiesi España S.A.U.Prescription not requiredDosage form: GEL, 5 g etofenamateActive substance: etofenamateManufacturer: Laboratorios Bial S.A.Prescription not required
Online doctors for FLOGOPATCH 70 MG MEDICATED ADHESIVE DRESSING
Discuss questions about FLOGOPATCH 70 MG MEDICATED ADHESIVE DRESSING, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






