
How to use Lixim
Leaflet attached to the packaging: patient information
Lixim, 70 mg, medicinal plaster
For use in adults
Etofenamate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor or pharmacist. See section 4.
- If after 7 days there is no improvement or the patient feels worse, they should contact a doctor.
Table of contents of the leaflet
- 1. What is Lixim and what is it used for
- 2. Important information before using Lixim
- 3. How to use Lixim
- 4. Possible side effects
- 5. How to store Lixim
- 6. Contents of the packaging and other information
1. What is Lixim and what is it used for
The active substance of this medicine, etofenamate, belongs to a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs), which have analgesic and anti-inflammatory effects.
Lixim is used for the short-term symptomatic treatment of local pain in acute, uncomplicated ankle sprains in adults.
If after 7 days there is no improvement or the patient feels worse, they should consult a doctor.
2. Important information before using Lixim
When not to use Lixim:
- if the patient is allergic to etofenamate or any of the other ingredients of this medicine (listed in section 6);
- if the patient is allergic to other non-steroidal anti-inflammatory drugs (NSAIDs, medicines used to treat pain and inflammation, such as acetylsalicylic acid or ibuprofen) with symptoms such as asthma, difficulty breathing (bronchospasm), rhinitis, swelling, or hives;
- if the patient has skin damage (e.g., skin abrasions, cuts, burns, open wounds), infection, or skin inflammation or eczema;
- in women in the last three months of pregnancy (see section "Pregnancy, breastfeeding, and fertility");
- on the eyes, mouth, or mucous membranes.
Warnings and precautions
If a skin rash occurs, the medicinal plaster should be removed immediately and treatment discontinued.
To minimize the risk of side effects, it is recommended to use the medicine for the shortest time necessary to control the symptoms.
A dressing should not be used to keep the plaster in place.
In patients with asthma or allergy, breathing difficulties (bronchospasm) may occur.
During treatment and for 2 weeks after removal of the plaster, the treated area should be avoided from direct exposure to sunlight or artificial UV radiation in a solarium to reduce the risk of photosensitivity.
It cannot be excluded that systemic side effects (affecting the whole body) may occur after using the medicinal plaster, if the medicine is used on large areas of skin (i.e., if more than one plaster is used - see section 3) and for a long time.
Although it is expected that systemic effects will be minimal, the medicinal plaster should be used with caution in patients with:
- renal, cardiac, or hepatic impairment
- active or history of gastrointestinal ulceration, inflammatory bowel disease, or tendency to bleeding.
Particular caution should be exercised when using non-steroidal anti-inflammatory drugs in elderly patients, as they are more susceptible to side effects.
Other medicines containing etofenamate or other non-steroidal anti-inflammatory drugs (NSAIDs) should not be used at the same time, either topically (on the skin) or orally.
Children and adolescents
Lixim should not be used in children and adolescents under 18 years of age, as the safety and efficacy of this medicine have not been established in this age group.
Lixim and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
If the medicine is used correctly, etofenamate is absorbed into the body in very small amounts, so the risk of interaction with other medicines is low.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before using this medicine.
This medicine should not be used in women in the third trimester of pregnancy.
In the first and second trimesters of pregnancy, the medicine can only be used on the advice of a doctor.
Small amounts of flufenamic acid, a metabolite of etofenamate, pass into breast milk in women who are breastfeeding. However, if the doctor considers it necessary, it is possible to use Lixim for a short time while breastfeeding. If Lixim is used during breastfeeding, it should not be applied to the breast area or to large areas of skin or for a long time.
Driving and using machines
Lixim has no or negligible influence on the ability to drive and use machines.
3. How to use Lixim
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
The recommended dose is:
Adults and elderly
Apply one (1) plaster every 12 hours (a total of 2 plasters per day), for no longer than 7 days.
Only one plaster can be used at a time.
For short-term use only.
Do not exceed the recommended dose.
Method of administration
For cutaneous use.
The plaster should only be applied to undamaged, healthy skin.
How to apply the plaster
The plaster should be applied to a dry, smooth skin surface. If the skin area where the plaster is to be
applied is sweaty or heavily hairy, the adhesion of the plaster may be reduced.
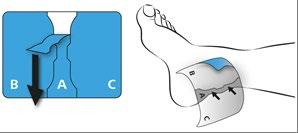
First, remove the protective layer from the center of the plaster (A) and press it firmly onto the skin.
Do not touch the adhesive side of the plaster with your fingers!
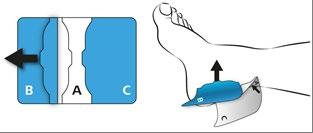
Hold the center of the plaster (A) with the other hand and remove part (B) of the protective layer by folding the plaster outwards and tearing off the protective layer (B) from the protruding edge (see arrow). After removing the protective layer, press the plaster onto the skin.
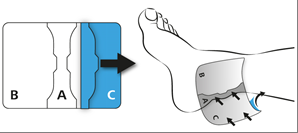
Remove part (C) of the protective layer in the same way as part (B). Press the plaster onto the skin.
Press the plaster onto the skin for at least 30 seconds to ensure proper adhesion.
After using each plaster, close the sachet tightly using the cord closure.
Duration of treatment
For short-term use only.
Lixim should be used for the shortest time necessary to relieve symptoms.
Do not use the plaster for more than 7 days. If after this time there is no improvement or symptoms worsen, consult a doctor.
Do not exceed the recommended treatment duration.
Overdose of Lixim
In case of overdose or accidental use by a child, seek medical attention immediately.
Missed application of Lixim
Do not use a double dose to make up for a missed dose.
If you have any further questions about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using this medicine and contact a doctor immediately if you experience:
- symptoms of an allergic reaction, such as asthma, unexplained wheezing or shortness of breath, itching, rhinitis, or skin rash.
- symptoms of hypersensitivity and skin reactions, such as redness, swelling, flaking, blistering, peeling, or skin ulceration.
The following side effects may occur:
Uncommon (may affect up to 1 in 100 people):
Skin inflammation, such as redness of the skin, itching, burning, skin rash, including with papules, pustules, or vesicles.
Rare (may affect up to 1 in 1,000 people):
Hypersensitivity reactions, local allergic reactions (contact dermatitis).
Very rare (may affect up to 1 in 10,000 people):
Skin swelling.
Frequency not known (cannot be estimated from the available data):
Photosensitivity (phototoxicity).
The risk of side effects increases if the plaster is used on a large skin area (e.g., using more than one plaster - see section 3) and for a long time.
Reporting side effects
If you experience any side effects, including any possible side effects not listed in the leaflet, you should inform your doctor or pharmacist. Side effects can be reported directly to the Department of Medicinal Product Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Lixim
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the sachet and carton after: Expiry date (EXP). The expiry date refers to the last day of the month.
Do not store above 30°C.
Used plasters should be folded in half, with the adhesive side inward, and disposed of safely.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Lixim contains
- The active substance is etofenamate. One medicinal plaster contains 70 mg of etofenamate.
- The other ingredients are:
Adhesive layer
Trimethylsililated polycondensate of alpha-hydro-omega-hydroxypoly (dimethylsiloxane) with dimeticone, macrogol 400, and purified olive oil.
Outer protective layer
Polyester.
Protective layer (to be removed)
Polyester coated with fluoropolymer.
What Lixim looks like and contents of the pack
Each plaster is 10 cm x 14 cm in size, made of white material, with a colorless, self-adhesive layer, and a removable protective foil.
Lixim is available in a carton containing a closed sachet. Each sachet contains 2, 5, or 7 medicinal plasters. The sachet is equipped with a cord closure to allow it to be closed after removing individual plasters.
Marketing authorization holder and manufacturer
Marketing authorization holder
Drossapharm Arzneimittel Handelsgesellschaf GmbH
Wallbrunnstrasse 24
79539 Lörrach
Germany
Manufacturer/Importer
mikle-pharm GmbH
Sandgasse 17
76829 Landau
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria/Germany:
Lixim 70 mg wirkstoffhaltiges Pflaster
Belgium:
Lixim 70 mg emplâtre médicamenteux
Lixim 70 mg wirkstoffhaltiges Pflaster
Lixim 70 mg pleister
Spain:
Flogopatch 70 mg apósito adhesivo medicamentoso
Hungary:
Lixim 70 mg gyógyszeres tapasz
Italy:
Dorsiflex
Poland:
Lixim
Portugal:
Fixplast 70 mg emplastro medicamentoso
Date of last revision of the leaflet:07.10.2022
- Country of registration
- Active substance
- Prescription requiredNo
- Importermikle-pharm GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LiximDosage form: Gel, 100 mg/gActive substance: etofenamateManufacturer: Laboratorios Basi - Industria Farmaceutica, S.A.Prescription not requiredDosage form: Gel, 100mg/gActive substance: etofenamatePrescription not requiredDosage form: Gel, 100 mg/gActive substance: etofenamateManufacturer: Mako Pharma Sp. z o.o. Medicofarma S.A.Prescription not required
Alternatives to Lixim in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Lixim in Spain
Alternative to Lixim in Ukraine
Online doctors for Lixim
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Lixim – subject to medical assessment and local rules.







