FINTEPLA 2.2 mg/ml ORAL SOLUTION

How to use FINTEPLA 2.2 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Fintepla 2.2 mg/ml Oral Solution
fenfluramine
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you or your child start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you or your child only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours or your child's.
- If you or your child get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Fintepla and what is it used for
- What you need to know before you or your child take Fintepla
- How to take Fintepla
- Possible side effects
- Storing Fintepla
- Contents of the pack and other information
1. What is Fintepla and what is it used for
Fintepla contains the active substance fenfluramine.
Fintepla is used as an adjunctive treatment for seizures in patients aged 2 years and older with a type of epilepsy called Dravet syndrome or Lennox-Gastaut syndrome. It may help reduce the number and severity of seizures.
The exact way Fintepla works is not known. However, it is thought to work by increasing brain activity of a substance called serotonin and the sigma 1 receptor, which may reduce seizures.
2. What you need to know before you or your child take Fintepla
Do not take Fintepla
- if you are allergic to fenfluramine or any of the other ingredients of this medicine (listed in section 6);
- if you have a heart problem such as 'valvular heart disease' or 'pulmonary arterial hypertension' (high blood pressure in the arteries of the lungs);
- if you have taken medicines called monoamine oxidase inhibitors (MAOIs) in the last 2 weeks.
Do not take Fintepla if any of the above apply to you. If you are not sure, consult your doctor, pharmacist, or nurse before taking Fintepla.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting Fintepla if:
- you or your child have glaucoma;
- you or your child have had thoughts of harming yourself or suicide;
- you or your child are taking a medicine called cyproheptadine, which is used to treat allergies or to improve appetite.
If any of the above apply to you (or if you are not sure), talk to your doctor or pharmacist before taking Fintepla.
Tests and checks
Before starting treatment with Fintepla, your doctor will check your heart with an echocardiogram (ECG). The doctor will check that the heart valves are not abnormal and that the blood pressure between the heart and lungs is not too high. Once you have started taking Fintepla, you will need to have an echocardiogram every 6 months for the first 2 years and then once a year. If you stop taking Fintepla, you will need to have an echocardiogram 3-6 months after the last dose.
Your doctor will also need to check your weight before and during treatment, as Fintepla may cause weight loss.
'Serotonin syndrome'
Tell your doctor or pharmacist before taking Fintepla if you or your child are taking medicines that may increase the level of serotonin in the brain. This is because taking these medicines with Fintepla may cause serotonin syndrome, a condition that can be life-threatening. Medicines that may increase serotonin levels are:
- 'triptans' (such as sumatriptan) used for migraine;
- MAOI medicines used for depression;
- SSRI or SNRI medicines used for depression and anxiety.
Be aware of the possible symptoms of serotonin syndrome, which include:
- agitation, seeing things that are not there (hallucinations), or fainting;
- circulatory and heart problems such as a fast heartbeat, changes in blood pressure, high body temperature, sweating;
- muscle tremors and lack of coordination;
- feeling sick or being sick and diarrhea.
Tell your doctor immediately if you experience any of these side effects.
Other medicines and Fintepla
Tell your doctor or pharmacist if you or your child are taking, have recently taken, or might take any other medicines. This is because Fintepla may affect how other medicines work. Also, some medicines may affect how Fintepla works.
Fintepla may cause drowsiness. You or your child may feel even more drowsy if you take medicines such as antidepressants or alcohol at the same time as Fintepla.
In particular, tell your doctor or pharmacist if you or your child are taking, have recently taken, or might take:
- stiripentol, a medicine for epilepsy, as your Fintepla dose may need to be reduced;
- 'triptans', MAOIs, SSRIs, or SNRIs (see 'Serotonin syndrome' above);
- carbamazepine, primidone, rifampicin, phenobarbital, and other barbiturates, phenytoin, or efavirenz, as your Fintepla dose may need to be increased.
You should also talk to a doctor or pharmacist if you or your child smoke, as you may need to have your Fintepla dose increased.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and using machines
Talk to your doctor about driving and using machines, or if you or your child plan to take part in activities such as cycling or other sports, as you may feel drowsy after taking this medicine.
Fintepla contains sodium ethyl p-hydroxybenzoate (E 215) and sodium methyl p-hydroxybenzoate (E 219)
May cause allergic reactions (possibly delayed).
Fintepla contains sulfur dioxide (E 220)
May rarely cause hypersensitivity reactions and bronchospasm.
Fintepla contains glucose
May be harmful to teeth.
If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
Fintepla contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per 12 ml, i.e., essentially 'sodium-free'.
3. How to take Fintepla
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
Your doctor, pharmacist, or nurse will calculate the dose volume up to the maximum recommended dose, using the following formula:
Weight (kg) x dose based on weight (mg/kg) ÷ 2.2 mg/ml = dose volume twice a day
The calculated dose should be rounded to the nearest graduated increment.
The following table should only be used as a check of the calculated dose volume. Table 1 does not replacethe need to calculate the specific dose volume.
Table 1: Range of dose volumes in ml for checking the calculation | |||||
Dose without concomitant STP* | Dose with concomitant STP** | ||||
Weight category | Starting dose | Day 7-13 | Day 14 and after | Starting dose | Day 7 and after |
0.1 mg/kg twice a day 0.2 mg/kg twice a day | 0.35 mg/kg twice a day | 0.1 mg/kg twice a day | 0.2 mg/kg twice a day | ||
3-5 kg | 0.2-0.3 ml | 0.3-0.5 ml | 0.5-0.8 ml | 0.2-0.3 ml | 0.3-0.5 ml |
5-7 kg | 0.3-0.4 ml | 0.5-0.7 ml | 0.8-1.2 ml | 0.3-0.4 ml | 0.5-0.7 ml |
7-10 kg | 0.4-0.5 ml | 0.7-1 ml | 1.2-1.6 ml | 0.4-0.5 ml | 0.7-1 ml |
10-15 kg | 0.5-0.7 ml | 1-1.4 ml | 1.6-2.4 ml | 0.5-0.7 ml | 1-1.4 ml |
15-20 kg | 0.7-1 ml | 1.4-1.9 ml | 2.4-3.2 ml | 0.7-1 ml | 1.4-1.9 ml |
20-30 kg | 1-1.4 ml | 1.9-2.8 ml | 3.2-4.8 ml | 1-1.4 ml | 1.9-2.8 ml |
30-38 kg | 1.4-1.8 ml | 2.8-3.5 ml | 4.8-6 ml (maximum dose) | 1.4-1.8 ml | 2.8-3.5 ml |
38-43 kg | 1.8-2 ml | 3.5-4 ml | 6 ml (maximum dose) | 1.8-2 ml | 3.5-4 ml (maximum dose) |
43-55 kg | 2-2.5 ml | 4-5 ml | 6 ml (maximum dose) | 2-2.5 ml | 4 ml (maximum dose) |
55-65 kg | 2.5-3 ml | 5-6 ml (maximum dose) | 6 ml (maximum dose) | 2.5-3 ml | 4 ml (maximum dose) |
65-86 kg | 3-4 ml | 6 ml (maximum dose) | 6 ml (maximum dose) | 3-4 ml (maximum dose) | 4 ml (maximum dose) |
86-130 kg | 4-6 ml (maximum dose) | 6 ml (maximum dose) | 6 ml (maximum dose) | 4 ml (maximum dose) | 4 ml (maximum dose) |
*Without concomitant STP: the maximum dose of 13 mg twice a day corresponds to 6 ml twice a day. **With concomitant STP: the maximum dose of 8.6 mg twice a day corresponds to 4 ml twice a day. |
How much to take
- Your doctor will tell you how many milliliters to take for each dose.
- Take the medicine twice a day.
- The doctor will start treatment with a low dose. This dose will be gradually increased according to how well the medicine works and how it affects you or your child.
- The maximum amount that can be taken is 6 ml twice a day.
- If you are taking stiripentol, the maximum amount that can be taken is 4 ml twice a day.
- Do not take more than the prescribed dose, as this may cause serious side effects.
How to take this medicine
- Take this medicine by mouth.
- Take the medicine with food or between meals.
- Fintepla oral solution is compatible with a ketogenic diet.
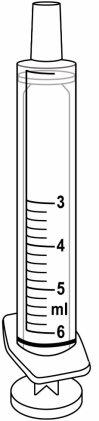
- The medicine is a liquid. Use the oral syringes provided to measure the dose, as described below.
- Use the green 3 ml syringe for doses up to 3.0 ml.
- Use the purple 6 ml syringe for doses between 3.2 and 6.0 ml.
- Fintepla oral solution is compatible with most enteral feeding tubes.
- To flush the feeding tube, fill the syringe with water and flush the tube. Repeat this process 3 times.
3 ml syringe – green | 6 ml syringe – purple |
|
|
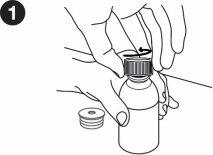
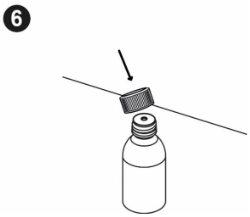
| Write the date you first opened the bottle on the box. You must put the bottle adapter on the first time you open it. The following instructions will show you how to put the adapter on. Inserting the bottle adapter: When you first open the bottle, you need to press the adapter into it. Wash and dry your hands. Remove the bottle adapter from its packaging. Place the bottle on a flat, hard surface. Open the bottle. |
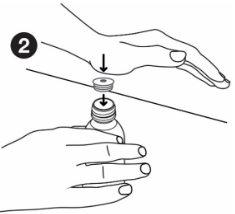
| Hold the bottle firmly. Align the adapter with the top of the bottle. Press the adapter into place with the palm of your hand until it is aligned with the top of the bottle. Leave the adapter in place after using the medicine. Screw the bottle cap back on without removing the adapter. |
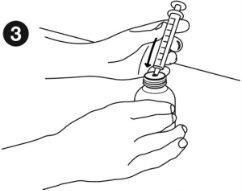
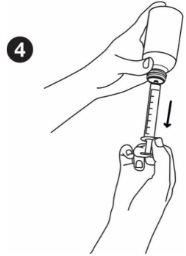
| Taking the medicine: Before measuring the dose, make sure the plunger is fully inserted into the oral syringe. Hold the medicine bottle firmly on a flat, hard surface. Insert the tip of the oral syringe into the bottle adapter until it cannot be pushed further. |
| Hold the syringe and bottle together and turn them upside down. Slowly pull out the plunger to draw up the correct dose. Hold the syringe and bottle together and turn them right side up. Holding the bottle firmly, gently pull the syringe out of the adapter. |
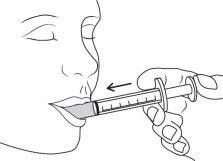
| Place the tip of the syringe inside the patient's mouth. Gently push the plunger until it reaches the bottom. A small amount will remain in the tip of the syringe. This is normal. Do not shoot the medicine to the back of the throat, as this could cause choking. |
| Put the cap back on the bottle and screw it on tightly. Always leave the adapter in place on the bottle. |
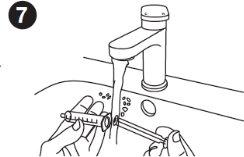
| Cleaning the syringe: Wash the syringe with clean water and let it air dry after each use. Wash the inside of the syringe and the plunger. Wash the syringe by using the plunger to draw in and push out clean water. Repeat this step several times. It is okay to separate the plunger from the syringe and wash each part. The syringe and plunger can be washed in a dishwasher. The syringe and plunger must be completely dry before the next use. |
If you or your child take more Fintepla than you should
Consult a doctor or go to the hospital immediately. Take the medicine bottle with you. The following effects may happen: agitation, drowsiness, or confusion, flushing or feeling hot, tremors or sweating.
If you or your child forget to take Fintepla
- Take it as soon as you remember. However, if it is almost time for the next dose, skip the missed dose.
- Do not take a double dose to make up for a forgotten dose.
If you or your child stop taking Fintepla
Do not stop taking Fintepla without talking to your doctor first. If your doctor decides to stop treatment with this medicine, they will ask you to gradually reduce the amount you take each day. Gradually reducing the dose will reduce the risk of having seizures and status epilepticus.
Between 3 and 6 months after the last dose of Fintepla, you or your child will need to have an echocardiogram.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Very Common: may affect more than 1 in 10 people
- Decreased appetite
- Somnolence
- Diarrhea
- Feeling tired, somnolent, or weak
Common: may affect up to 1 in 10 people
- bronchitis
- abnormal behavior
- rapid mood changes
- aggressiveness
- agitation
- insomnia
- tremor of hands, arms, or feet
- problems coordinating movements, walking, and maintaining balance
- decreased muscle tone
- seizures
- prolonged seizures (status epilepticus)
- lethargy
- weight loss
- constipation
- excessive salivation
- vomiting
- skin rash
low blood sugar level
- elevated prolactin levels in blood
Frequency Not Known(frequency cannot be estimated from available data):
- valvular heart disease
- irritability
- serotonin syndrome
- high blood pressure in the pulmonary arteries (pulmonary arterial hypertension)
Inform your doctor or nurse if you notice any of the adverse effects listed above.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Fintepla
- Keep this medicine out of sight and reach of children.
- Do not use this medicine after the expiration date shown on the box and vial after CAD. The expiration date is the last day of the month indicated.
- Do not refrigerate or freeze.
- It must be used before 3 months have passed since the first opening of the vial.
- Wash the syringe after each use.
- If a syringe is damaged or lost, or if you cannot read the dose marks it presents, use another syringe for oral administration from those included in the package or consult your pharmacist.
- Medicines should not be thrown away through drains or into the trash. Ask your pharmacist how to dispose of containers and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Fintepla Composition
The active ingredient is fenfluramine. Each milliliter contains 2.2 mg of fenfluramine (as fenfluramine hydrochloride).
The other components are:
- Sodium ethyl p-hydroxybenzoate (E 215)
- Sodium methyl p-hydroxybenzoate (E 219)
- Sucralose (E 955)
- Hydroxyethylcellulose (E 1525)
- Monosodium phosphate (E 339)
- Disodium phosphate (E 339)
- Cherry flavor powder:
- Acacia (E 414)
- Glucose (from corn)
- Ethyl benzoate
- Natural flavor preparations
- Natural flavoring substances
- Flavoring substances
- Maltodextrin (corn)
- Sulfur dioxide (E 220)
- Potassium citrate (E 332)
- Citric acid monohydrate (E 330)
- Water for injectable preparations
Product Appearance and Package Contents
- Fintepla oral solution is supplied as a clear, colorless, slightly viscous liquid with a cherry flavor.
- The solution is presented in a white vial with a child-resistant and tamper-evident closure.
- Each box contains:
- Vial with 60 ml of oral solution, a vial adapter, two 3 ml oral syringes with 0.1 ml graduations, and two 6 ml syringes with 0.2 ml graduations.
- Vial with 120 ml of oral solution, a vial adapter, two 3 ml oral syringes with 0.1 ml graduations, and two 6 ml syringes with 0.2 ml graduations.
- Vial with 250 ml of oral solution, a vial adapter, two 3 ml oral syringes with 0.1 ml graduations, and two 6 ml syringes with 0.2 ml graduations.
- Vial with 360 ml of oral solution, a vial adapter, two 3 ml oral syringes with 0.1 ml graduations, and two 6 ml syringes with 0.2 ml graduations.
- It is possible that in your country, only some package sizes are marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
UCB Pharma S.A.,
Allée de la Recherche 60,
B-1070 Bruxelles,
Belgium
Manufacturer:
Millmount Healthcare Ltd,
Millmount Site, Block 7,
City North Business Campus,
Stamullen,
Co. Meath,
K32 YD60,
Ireland
You can request more information about this medicine by contacting the local representative of the Marketing Authorization Holder:
België/Belgique/Belgien UCB Pharma S.A./NV Tél/Tel: + 32 / (0)2 559 92 00 | Lietuva UAB Medfiles Tel: +370 5 246 16 40 |
| Luxembourg/Luxemburg UCB Pharma SA/NV Tél/Tel: + 32 / (0)2 559 92 00 (Belgique/Belgien) |
Ceská republika UCB s.r.o. Tel: + 420 221 773 411 | Magyarország UCB Magyarország Kft. Tel.: + 36-(1) 391 0060 |
Danmark UCB Nordic A/S Tlf: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Deutschland UCB Pharma GmbH Tel: + 49 /(0) 2173 48 4848 | Nederland UCB Pharma B.V. Tel.: + 31 / (0)76-573 11 40 |
Eesti OÜ Medfiles Tel: +372 730 5415 | Norge UCB Nordic A/S Tlf: + 47 / 67 16 5880 |
Ελλάδα UCB Α.Ε. Τηλ: + 30 / 2109974000 | Österreich UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
España UCB Pharma, S.A. Tel: + 34 / 91 570 34 44 | Polska UCB Pharma Sp. z o.o. / VEDIM Sp. z o.o. Tel: + 48 22 696 99 20 |
France UCB Pharma S.A. Tél: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 21 302 5300 |
Hrvatska Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 | Ireland UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 |
România UCB Pharma Romania S.R.L. Tel: + 40 21 300 29 04 | Slovenija Medis, d.o.o. Tel: + 386 1 589 69 00 |
Ísland Vistor hf. Simi: + 354 535 7000 | Slovenská republika UCB s.r.o., organizačná zložka Tel: + 421 (0) 2 5920 2020 |
Italia UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Suomi/Finland UCB Pharma Oy Finland Puh/Tel: + 358 9 2514 4221 |
Κύπρος Lifepharma (Z.A.M.) Ltd Τηλ: + 357 22 056300 | Sverige UCB Nordic A/S Tel: + 46 / (0) 40 294 900 |
Latvija Medfiles SIA Tel: . +371 67 370 250 |
Date of Last Revision of this Prospectus:
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites about rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FINTEPLA 2.2 mg/ml ORAL SOLUTIONDosage form: TABLET, 100 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: TABLET, 200 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: TABLET, 25 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription required
Online doctors for FINTEPLA 2.2 mg/ml ORAL SOLUTION
Discuss questions about FINTEPLA 2.2 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions