EVENITY 105 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN

How to use EVENITY 105 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
EVENITY 105 mg solution for injection in pre-filled pen
romosozumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- You will be given a patient alert card that contains important safety information that you need to know before and during treatment with EVENITY.
Contents of the pack
- What is EVENITY and what is it used for
- What you need to know before you use EVENITY
- How to use EVENITY
- Possible side effects
- Storage of EVENITY
- Contents of the pack and other information
1. What is EVENITY and what is it used for
What is EVENITY
Evenity contains the active substance romosozumab, a medicine that helps to strengthen bones and reduce the risk of fractures.
What is EVENITY used for
EVENITY is used to treat severe osteoporosis in postmenopausal women who are at high risk of fractures.
Osteoporosis is a disease that makes bones fragile and brittle. Many patients with osteoporosis do not show any symptoms, but they may be at a higher risk of fractures.
How EVENITY works
EVENITY is a monoclonal antibody. A monoclonal antibody is a type of protein that has been designed to recognize and bind to specific proteins in the body. EVENITY binds to a protein called sclerostin. By binding to it and blocking its activity, EVENITY:
- helps to form new bone and
- slows down the loss of existing bone.
This makes bones stronger and reduces the risk of fractures.
2. What you need to know before you use EVENITY
Do not use EVENITY
- if you are allergic to romosozumab or any of the other ingredients of this medicine (listed in section 6);
- if you have low levels of calcium in your blood (hypocalcemia). Your doctor will be able to tell you if your levels are too low;
- if you have a history of heart attack or stroke.
Do not use EVENITY if you are in any of the above situations. If you are not sure, talk to your doctor or pharmacist before using EVENITY.
Warnings and precautions
Talk to your doctor or pharmacist and discuss your medical history before you start using EVENITY.
Heart attack and stroke
Heart attack and stroke have been reported in people taking EVENITY.
Seek medical attention immediatelyif you experience:
- chest pain, difficulty breathing;
- headache, numbness or weakness in the face, arms or legs, difficulty speaking, changes in vision, loss of balance.
Your doctor will carefully assess the risk of cardiovascular problems before allowing you to start treatment with EVENITY. Tell your doctor if you know you have a high risk of cardiovascular problems such as established cardiovascular disease, high blood pressure, high levels of fat in the blood, diabetes, smoking or kidney problems.
Low levels of calcium in the blood
EVENITY may cause low levels of calcium in the blood.
Tell your doctorif you notice:
- muscle spasms, twitches or cramps;
- numbness or tingling in the fingers or toes, or around the mouth.
Your doctor may prescribe calcium and vitamin D to help you prevent low levels of calcium in the blood before you start treatment and while you are taking EVENITY. Take calcium and vitamin D as directed by your doctor. Tell your doctor if you have or have had severe kidney problems, kidney failure or have needed dialysis, as this may increase your risk of having low levels of calcium in the blood if you do not take calcium supplements.
Severe allergic reactions
People taking EVENITY may have severe allergic reactions.
Seek medical attention immediatelyif you experience:
- swelling of the face, mouth, throat, hands, feet, ankles, lower legs (angioedema), or hives;
- acute skin rash with multiple red/pink round spots with a blister or crust in the center (erythema multiforme);
- difficulty swallowing or breathing.
Problems in the mouth, teeth or jaw
In patients taking EVENITY, a rare side effect (may affect up to 1 in 1,000 people) called osteonecrosis of the jaw (ONJ, bone damage in the jaw) has been reported. ONJ can also occur after stopping treatment. It is important to try to prevent ONJ from occurring, as it can be a painful condition that can be difficult to treat. To reduce the risk of ONJ, you should take some precautions.
Before you receive EVENITY, tell your doctor or nurse if:
- you have any problems with your mouth or teeth, such as poor dental health, gingivitis or are scheduled for a tooth extraction;
- you do not receive regular dental care or have not had a dental check-up for a long time;
- you are a smoker (as this can increase the risk of dental problems);
- you have previously received treatment with bisphosphonates (used to treat or prevent bone disorders such as osteoporosis);
- you are taking medicines called corticosteroids (such as prednisolone or dexamethasone);
- you have cancer.
Your doctor may ask you to have a dental examination before you start treatment with EVENITY.
During treatment, you should maintain good oral hygiene and have regular dental check-ups. If you wear dentures, you should make sure they fit properly. If you are receiving dental treatment or are going to have a dental procedure (such as a tooth extraction), tell your doctor about your dental treatment and inform your dentist that you are receiving treatment with EVENITY.
Get in touch with your doctor and dentist immediatelyif you have any problems with your mouth or teeth, such as:
- loosening of teeth;
- pain or swelling;
- mouth ulcers that do not heal;
- discharge.
Atypical fractures of the thigh bone
In people who have used EVENITY, atypical fractures of the thigh bone have rarely occurred (may affect up to 1 in 1,000 people) due to minor or no trauma. These types of fractures were often preceded by warning signs such as pain in the thigh or groin for several weeks before the fracture occurred. It is not known if EVENITY caused these unusual fractures. Tell your doctor or pharmacist if you experience any new or unusual pain in your hip, groin or thigh.
Children and adolescents
The use of romosozumab has not been studied in children and adolescents and is not approved for use in pediatric patients (aged <18 years).< p>
Other medicines and EVENITY
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy and breastfeeding
EVENITY is expected to be used only to treat postmenopausal women.
EVENITY should not be used in women of childbearing potential, during pregnancy or breastfeeding. It is not known if EVENITY can harm the fetus or the baby. Talk to your doctor if you have any questions.
Driving and using machines
EVENITY is not expected to have any significant effect on the ability to drive or use machines.
EVENITY contains sodium and polysorbate 20
This medicine contains 0.070 mg of polysorbate 20 in each pre-filled pen. Polysorbates may cause allergic reactions. Tell your doctor if you are allergic to any of these ingredients.
This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially sodium-free.
3. How to use EVENITY
Treatment with EVENITY will be started and supervised by specialized doctors with experience in the treatment of osteoporosis. Follow your doctor’s instructions for administration of this medicine exactly. If you are unsure, talk to your doctor.
Only a person with the appropriate training should give the injection.
How much to use
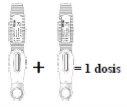
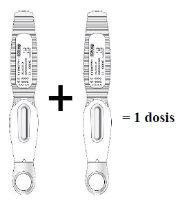
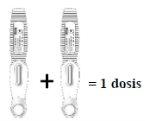
- The recommended dose of EVENITY is 210 mg.
- Since a pre-filled pen contains 105 mg of the active substance romosozumab in 1.17 ml of solution (90 mg/ml), 2 pre-filled pens should be used for each dose. The second injection should be given immediately after the first, but in a different injection site.
- This should be done once a month for 12 months.
How to use
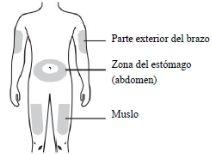
- EVENITY should be injected under the skin (subcutaneous injection).
- EVENITY should be injected into the abdomen or thigh. The outer aspect of the arm can also be used as an injection site, but only if someone else is giving the injection.
- If the same injection site is to be used for the second injection, a different point should be used.
- EVENITY should not be injected into areas where the skin is tender, bruised, red or hardened.
It is important that you read the Instructions for useto get information on how to use the pre-filled pen EVENITY.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
If you use more EVENITY than you should
If you have used more EVENITY than you should by mistake, talk to your doctor or pharmacist.
If you miss or cannot use EVENITY at the scheduled time
If you miss a dose of EVENITY, talk to your doctor as soon as possible to schedule another dose. From then on, the next dose should be given at least one month after the date of the last dose.
If you stop treatment with EVENITY
If you are considering stopping treatment with EVENITY, discuss this with your doctor. Your doctor will tell you how long you should receive treatment with EVENITY.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
Talk to your doctor about the need to switch to another treatment for osteoporosis after the end of your treatment with EVENITY.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek medical attention immediatelyif you experience any of the following symptoms of heart attackor stroke(uncommon: may affect up to 1 in 100 people):
- chest pain, difficulty breathing;
- headache, numbness or weakness in the face, arms or legs, difficulty speaking, changes in vision, loss of balance.
Seek medical attention immediatelyif you experience any of the following symptoms of severe allergic reaction(rare: may affect up to 1 in 1,000 people):
- swelling of the face, mouth, throat, hands, feet, ankles, lower legs (angioedema), or hives;
- acute skin rash with multiple red/pink round spots with a blister or crust in the center (erythema multiforme);
- difficulty swallowing or breathing.
Tell your doctorif you notice any of the following symptoms of low levels of calciumin the blood (hypocalcemia) (uncommon: may affect up to 1 in 100 people):
- muscle spasms, twitches or cramps;
- numbness or tingling in the fingers or toes, or around the mouth.
See also section 2 “What you need to know before you use EVENITY”.
Other side effects may include:
Very common side effects(may affect more than 1 in 10 people):
- common cold;
- joint pain.
Common side effects(may affect up to 1 in 10 people):
- rash, skin inflammation;
- headache;
- inflammation of the sinuses;
- neck pain;
- muscle spasms;
- redness or pain at the injection site.
Uncommon side effects(may affect up to 1 in 100 people):
- hives (urticaria);
- cataracts.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of EVENITY
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after “EXP/CAD”. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C - 8°C). Do not freeze.
Once you have removed the carton with the pre-filled pens from the refrigerator to use them, you should not put them back in the refrigerator, but you can store them at room temperature (up to 25°C) for 30 days. If you do not use them within this period, the product should be discarded.
Keep the pre-filled pen in the outer carton to protect it from light.
Check the solution visually before use. You should not use the solution if it has changed color, is cloudy or contains flakes or particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help to protect the environment.
6. Container Contents and Additional Information
EVENITY Composition
- The active ingredient is romosozumab. Each prefilled syringe contains 105 mg of romosozumab in 1.17 ml of solution (90 mg/ml).
- The other components are calcium acetate, glacial acetic acid, sodium hydroxide (for pH adjustment), sucrose, polysorbate 20, and water for injectable preparations. See section 2 "EVENITY contains sodium".
Product Appearance and Container Contents
EVENITY is a clear to opalescent, colorless to yellowish injectable solution presented in a single-use, prefilled syringe. The syringe inside the pen is made of plastic and has a stainless steel needle.
Container with 2 prefilled syringes.
Multiple container with 6 (3 containers of 2) prefilled syringes.
Only some pack sizes may be marketed.
Marketing Authorization Holder
UCB Pharma S.A.,
Allée de la Recherche 60,
B-1070 Brussels, Belgium
Manufacturer
Amgen Europe B.V., Minervum 7061, 4817 ZK Breda, Netherlands
You can request more information about this medicine by contacting the local representative of the marketing authorization holder.
België/Belgique/Belgien UCB Pharma SA/NV Tél/Tel: + 32 / (0)2 559 92 00 | Lietuva UCB Pharma Oy Finland Tel: + 358 9 2514 4221 |
| Luxembourg/Luxemburg UCB Pharma SA/NV Tél/Tel: + 32 / (0)2 559 92 00 |
Ceská republika UCB s.r.o. Tel: + 420 221 773 411 | Magyarország UCB Magyarország Kft. Tel.: + 36-(1) 391 0060 |
Danmark UCB Nordic A/S Tlf: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Deutschland UCB Pharma GmbH Tel: + 49 /(0) 2173 48 4848 | Nederland UCB Pharma B.V. Tel.: + 31 / (0)76-573 11 40 |
Eesti UCB Pharma Oy Finland Tel: + 358 9 2514 4221 | Norge UCB Nordic A/S Tlf: + 45 / 32 46 24 00 |
Ελλάδα UCB Α.Ε. Τηλ: + 30 / 2109974000 | Österreich UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
España UCB Pharma, S.A. Tel: + 34 / 91 570 34 44 | Polska UCB Pharma Sp. z o.o. Tel: + 48 22 696 99 20 |
France UCB Pharma S.A. Tél: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 / 21 302 5300 |
Hrvatska Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 | România UCB Pharma Romania S.R.L. Tel: + 40 21 300 29 04 |
Ireland UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 | Slovenija Medis, d.o.o. Tel: + 386 1 589 69 00 |
Ísland Vistor hf. Simi: + 354 535 7000 | Slovenská republika UCB s.r.o., organizačná zložka Tel: + 421 (0) 2 5920 2020 |
Italia UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Suomi/Finland UCB Pharma Oy Finland Puh/Tel: + 358 9 2514 4221 |
Κύπρος Lifepharma (Z.A.M.) Ltd Τηλ: + 357 22 34 74 40 | Sverige UCB Nordic A/S Tel: + 46 / (0) 40 29 49 00 |
Latvija UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Somija) |
Date of Last Revision of this Leaflet: MM/YYYY.
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
Back to Page to View Instructions for Use
--------------------------------------------------------------------------------------------------------------------
INSTRUCTIONS FOR USE FOR THE INJECTION OF EVENITY USING A PREFILLED SYRINGE
Inject two prefilled syringes, one immediately after the other, to administer a full dose
The instructions included below explain how to use the prefilled syringe to inject EVENITY.
- Read these instructions carefully and follow them step by step.
- If you have any questions or are unsure about the injection procedure, contact a doctor or pharmacist.
- It is essential to ensure that only a person with the proper training administers the injection.
- The prefilled syringe is also called the "medicine".
Guide to the Parts: Prefilled Syringe
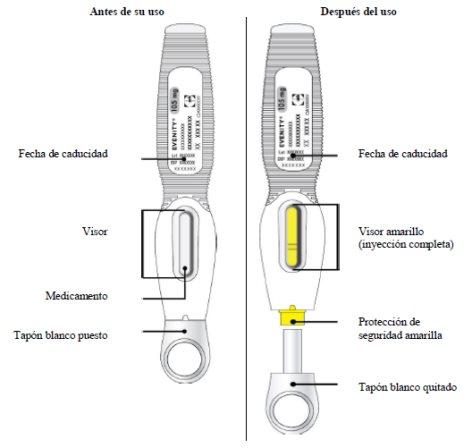
| Read this before injecting the medicine. Your doctor has prescribed a dose of 210 mg every month: To receive the full dose,two prefilled syringes with 105 mg eachmust be injected, one immediately after the other. |
|
Step 1: Prepare
A
- Remove the box containing the two prefilled syringes from the refrigerator.
- Your prefilled syringes should be left outside the refrigerator to reach room temperature (up to 25 °C) for at least 30 minutesbefore injection (they should not be heated in any other way). This will make the injection more comfortable.
- Open the box and gather all the materials you need for the injection (as listed in Step B)
- Wash your hands thoroughly.
- Lift the prefilled syringes straight up to remove them from the box. Do not remove the white caps from the syringes yet.
- Do not shake the prefilled syringes.
- Check the medicine through the view. The medicine should be a clear to opalescent, colorless to yellowish solution.
- The prefilled syringe should not be used if the solution has changed color, is cloudy, or contains flakes or particles.
- Bubbles may be visible. Subcutaneous injection (under the skin) of a solution containing air bubbles is harmless.
- Do not use the prefilled syringe if:
- It has fallen;
- The white cap is not on or is not firmly attached;
- The seal is not on or is broken, or if any part appears cracked or broken.
In these cases, use a new prefilled syringe and contact your doctor as soon as possible.
BOn a clean and well-lit work surface, place:
|
|
CPrepare and clean the skin where you will inject the medicine. You can choose from:
|
|
- The second injection should be administered in a different location from the first injection. If you want to use the same injection site, make sure it is not exactly the same point of injection.
- You should not inject into areas where the skin is painful, bruised, red, hardened, scarred, or shows lesions or thick, red, or scaly patches.
- Clean the area where you will inject with an alcohol swab. Let the skin dry before injecting.
- Do not touch this area again before injecting.
Step 2: Prepare
D |
|
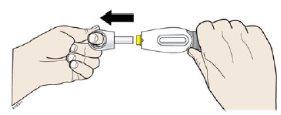
- Do not twist or bend the white cap.
- Discard the white cap in the special waste container. Do not put the white cap back on the prefilled syringe.
- Although it is out of sight, the needle tip is now exposed. Do not try to touch the needle, as this could activate the prefilled syringe. It is normal to see a drop of liquid on the needle tip (within the yellow safety protector).
EStretch or pinch the injection site to create a firm surface.
Stretch
- Stretch the skin well by moving your thumb and fingers in opposite directions, creating a 5 cm wide area.
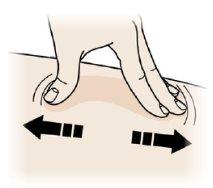
O
Pinch
- Pinch the skin firmly between your thumb and fingers to create an area of approximately 5 cm wide
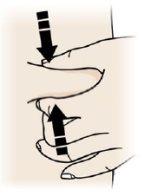
- Important:Keep the skin stretched or pinched during the injection.
Step 3: Inject
F |
|
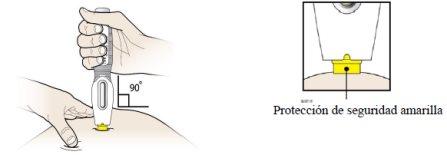
G |
|
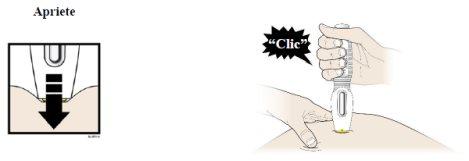
H |
|
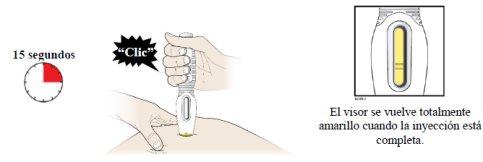
- You can now remove the prefilled syringe by carefully pulling it straight out and away from the skin.
- Important:When you remove the prefilled syringe, if the view has not turned completely yellow or if it seems that the medicine is still being injected, this indicates that the full dose has not been administered. You should inform your healthcare professional as soon as possible.
- After removing the prefilled syringe from the skin, the needle will be automatically covered. Do not try to touch the needle.
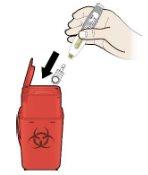
Step 4: Dispose
I |
|
|
|
Step 5: Examine the Injection Site
J | If there is bleeding, use a piece of cotton or gauze and press gently on the injection site for a few seconds. Do not rub the injection site. You can cover the injection site with a band-aid if necessary. |
Step 6: Repeat the Process to Administer the Second Injection and Achieve the Full Dose
K | Repeat all the steps from Step C with the second prefilled syringe to inject the full dose. The second injection should be administered in a different location from the first injection. If you want to use the same injection site, make sure it is not exactly the same point of injection. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EVENITY 105 mg SOLUTION FOR INJECTION IN A PRE-FILLED PENDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription requiredDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription requiredDosage form: INJECTABLE, 60 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription required
Online doctors for EVENITY 105 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Discuss questions about EVENITY 105 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions