EPIDYOLEX 100 mg/ml ORAL SOLUTION


How to use EPIDYOLEX 100 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Epidyolex 100 mg/ml Oral Solution
cannabidiol
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you or your patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Epidyolex and what is it used for
- What you or your patient need to know before you start taking Epidyolex
- How you or your patient should take Epidyolex
- Possible side effects
- Storing Epidyolex
- Contents of the pack and further information
1. What is Epidyolex and what is it used for
Epidyolex contains cannabidiol, a medicine that can be used to treat epilepsy, a condition that causes seizures or convulsions.
Epidyolex is used in combination with clobazam or with clobazam and other anti-epileptic medicines to treat seizures that occur due to two rare diseases, Dravet syndrome and Lennox-Gastaut syndrome. It can be used in adults, adolescents, and children from 2 years of age.
Epidyolex is also used with other anti-epileptic medicines to treat seizures associated with a genetic disease called tuberous sclerosis complex (TSC). It can be used in adults, adolescents, and children from 2 years of age.
2. What you or your patient need to know before you start taking Epidyolex
Do not take Epidyolex
- if you are allergic to cannabidiol or any of the other ingredients of this medicine (listed in section 6).
- if your doctor determines that your liver blood tests show abnormalities.
Warnings and precautions
Talk to your doctor or pharmacist before taking Epidyolex or during treatment if:
- you have or have had liver problems, as your doctor may need to change the dose of Epidyolex or may decide that Epidyolex is not suitable for you.
Your doctor may perform blood tests to examine your liver before you start taking this medicine or during treatment, as Epidyolex may cause liver problems. If your liver is not working properly, treatment may need to be stopped.
- you notice unusual changes in your mood or behavior, or if you have thoughts of self-harm or suicide. Contact your doctor or go to the hospital immediately(see section 4).
- Epidyolex may cause drowsiness. Do not drive, use machines, or engage in activities that require you to be alert and have proper control of your body, such as cycling, until you know how Epidyolex affects you.
- you stop taking Epidyolex suddenly (see section 3).
- you have more frequent seizures or a severe seizure while taking Epidyolex. Contact your doctor or go to the hospital immediately.
- you lose weight or are unable to gain weight. Your doctor will monitor your weight and assess the continuation of treatment with Epidyolex.
Children and adolescents
Epidyolex is not recommended in children under 2 years of age.
Other medicines and Epidyolex
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Taking Epidyolex with certain other medicines may cause side effects, may affect the way other medicines work, or may affect the way Epidyolex works. Do not start or stop treatment with other medicines without talking to your doctor or pharmacist.
Tell your doctor if you are taking any of the following medicines, as your dose may need to be adjusted:
- other medicines for epilepsy, such as carbamazepine, clobazam, lamotrigine, lorazepam, phenytoin, stiripentol, and valproate used to treat seizures;
- other medicines for the treatment of TSC, including everolimus and tacrolimus
- medicines used to treat gastroesophageal reflux (heartburn or acid reflux), such as omeprazole;
- mitotane (a medicine used to treat tumors in the adrenal gland);
- morphine or diflunisal (medicines used to treat pain);
- efavirenz (a medicine used to treat HIV/AIDS);
- theophylline (a medicine used to treat asthma);
- caffeine (a medicine for babies who need help breathing);
- propofol (an anesthetic used for people undergoing surgery);
- simvastatin, fenofibrate, gemfibrozil (medicines used to lower cholesterol/lipids);
- enzalutamide (a medicine used to treat prostate cancer);
- bupropion (a medicine used to help stop smoking or treat obesity);
- St. John's Wort (Hypericum perforatum), a herbal medicine used to treat mild anxiety problems;
- medicines used to treat bacterial infections, such as rifampicin, clarithromycin, and erythromycin.
Using Epidyolex with food
Always take Epidyolex as directed by your doctor and consistently with or without food, including ketogenic meals (such as ketogenic diets). If you take Epidyolex with food, try to keep your meals similar, if possible. (See also section 3: How to take Epidyolex.)
Pregnancy, breastfeeding, and fertility
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. You should not take Epidyolex during pregnancy unless your doctor decides that the benefits outweigh the possible risks. You should not breastfeed while taking Epidyolex, as Epidyolex may be present in breast milk.
Driving and using machines
Talk to your doctor about driving, using machines, or when children engage in activities such as cycling or other sports, as you or your patient may feel drowsy after taking the medicine.
Do not drive, do not use machinery, and do not engage in activities that require you to be alert and have proper control of your body until it is determined that your ability to perform these activities is not affected.
Epidyolex contains sesame oil, alcohol (ethanol), and flavoring components, including benzyl alcohol.
Epidyolex contains refined sesame oil, which can rarely cause severe allergic reactions.
Each ml of Epidyolex contains 79 mg of ethanol, which is equivalent to 10% v/v anhydrous ethanol, i.e., up to 691.3 ml of ethanol per maximum single dose of Epidyolex (12.5 mg/kg) for a 70 kg adult (9.9 mg of ethanol/kg). For a 70 kg adult, this is equivalent to 17 milliliters (ml) of beer or 7 ml of wine per dose. The small amount of alcohol in this medicine does not produce any noticeable effect.
This medicine contains 0.0003 mg/ml of benzyl alcohol, which is equivalent to 0.0026 mg per maximum dose of Epidyolex (Epidyolex 12.5 mg/kg per dose for a 70 kg adult). Benzyl alcohol may cause allergic reactions.
This product should not be used for more than one week in children (under 3 years of age) unless directed by your doctor or pharmacist.
Talk to your doctor or pharmacist if you are pregnant or breastfeeding. This is because large amounts of benzyl alcohol can build up in your body and cause side effects (called "metabolic acidosis").
Talk to your doctor or pharmacist if you have liver or kidney disease. This is because large amounts of benzyl alcohol can build up in your body and cause side effects (called "metabolic acidosis").
3. How you or your patient should take Epidyolex
Follow the instructions for administering this medicine exactly as directed by your doctor.
If you are unsure, talk to your doctor or pharmacist again.
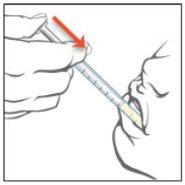
Epidyolex is an oral solution (a liquid to be swallowed). Your doctor or pharmacist will tell you how much Epidyolex (number of ml) to take each day, how many times a day to take it, and which oral syringe to use for your dose (1 ml or 5 ml).
Your doctor will calculate the dose based on your body weight. You may start treatment with a low dose that your doctor will gradually increase over time. Contact your doctor if you are unsure about your dose or if you think it may need to be changed.
Taking Epidyolex with food may increase the amount of medicine your body absorbs. Try to take Epidyolex consistently with or without food, and according to your daily routine so that you get the same effect each time. If you take Epidyolex with food, try to eat similar meals (e.g., with a similar fat content), if possible.
If necessary, Epidyolex can be given through a nasogastric or gastrostomy tube. Your doctor will provide instructions on this. Talk to your doctor or pharmacist if you are unsure how to do this.
Tell your doctor if you have liver problems, as your doctor may need to adjust the dose.
Do not reduce the dose or stop treatment with this medicine unless your doctor tells you to.
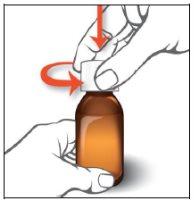
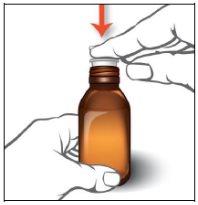
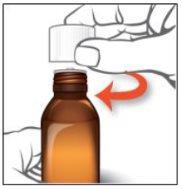
Instructions for oral use of Epidyolex
The pack of 1 bottle contains the following:
- oral solution bottle of Epidyolex
- a plastic bag with two 1 ml oral syringes and a bottle adapter
- a plastic bag with two 5 ml oral syringes and a bottle adapter
The pack of 3 bottles contains the following:
- three oral solution bottles of Epidyolex
- a plastic bag with two 1 ml oral syringes and a bottle adapter
- a plastic bag with two 5 ml oral syringes and two bottle adapters
A spare syringe of each size is included in the pack in case the first one is damaged or lost. For the pack of three bottles, all three adapters from both syringe packs are needed.
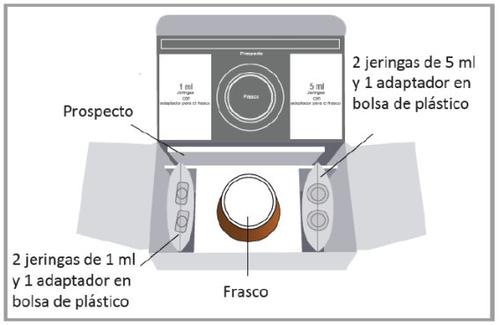
The image above is for illustrative purposes only.
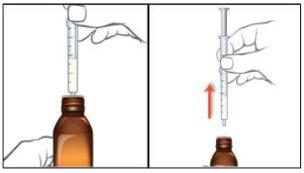
- Open the bag that contains the correct oral syringe to measure your dose.
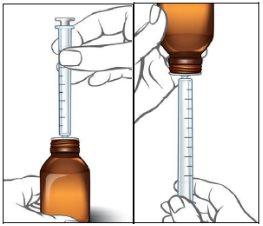
- If your dose is 1 ml (100 mg) or less, use the smaller 1 ml syringe.
- If your dose is more than 1 ml (100 mg), use the larger 5 ml syringe.
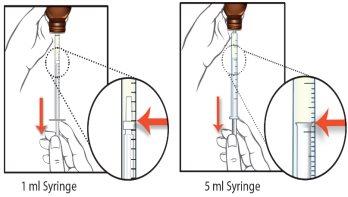
- If your dose is more than 5 ml (500 mg), you will need to use the larger 5 ml syringe more than once. In this case, keep track of how many times you fill the syringe (e.g., by marking each 5 ml dose respectively) to ensure you take the correct dose.
It is important to use the correct oral syringe to measure your dose. Your doctor or pharmacist will tell you which syringe to use based on the dose prescribed for you.
According to the doctor's or pharmacist's instructions, the bag containing the other syringes should be discarded, and the adapter from the pack should be discarded unless your doctor or pharmacist tells you to keep the two syringes until the final dose is reached. If you are prescribed a pack of 3 bottles, you should keep all three adapters.
|
|
|
|
|
|
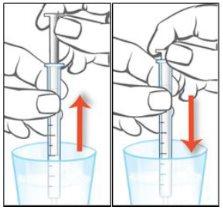
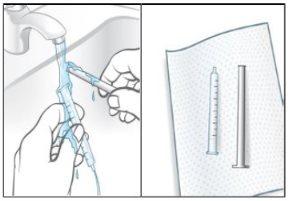
If there are any bubbles in the syringe, return the liquid to the bottle by holding it upside down and repeat step 5 until the bubble is removed. |
|
|
|
If the dose is more than 5 ml, repeat steps 4 to 7 to take the remaining dose with the 5 ml oral syringe. |
|
|
|
|
|
Shake off the water from the parts and let them air dry until the next use. Make sure the oral syringe is completely dry before its next use; otherwise, if water enters the bottle, it may cause the solution to become cloudy. If the solution in the bottle becomes cloudy, this does not change its effectiveness. Continue to use the medicine as usual. |
|
If you or your patient take more Epidyolex than you should
If you have taken more Epidyolex than you should, talk to your doctor or pharmacist immediately, or contact the emergency department of your nearest hospital and take the medicine with you.
Signs of taking more Epidyolex than you should include diarrhea and drowsiness.
If you or your patient forget to take Epidyolex
If you forget to take a dose, do not take a double dose to make up for the forgotten dose. Take the next dose when it is due. If you forget several doses, talk to your doctor about what dose you should take.
If you or your patient stop taking Epidyolex
Do not reduce the dose or stop treatment with Epidyolex without talking to your doctor first. Stopping this treatment suddenly can increase your seizures. Your doctor will explain how to stop treatment with Epidyolex gradually.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
The following adverse effects may be very serious:
Frequent Adverse Effects(may affect more than 1 in 100 people):
- In patients treated with Epidyolex, elevated levels of liver enzymes (increases in transaminases) have been reported in blood tests, which may be a sign of liver damage.
Adverse Effects of Unknown Frequency(cannot be estimated from the available data):
- People taking this medicine may have thoughts that lead them to self-harm or suicide. If you experience these thoughts at any time, contact your doctor.
You may experience the following adverse effects with this medicine. Inform your doctor if you notice any of the following:
Very Frequent Adverse Effects(may affect more than 1 in 10 people):
- drowsiness
- diarrhea
- decreased appetite
- fever
- vomiting
- fatigue
Frequent Adverse Effects(may affect more than 1 in 100 people):
- seizures
- displaying bad temper (irritability, aggression)
- skin rash
- lack of energy
- cough
- pneumonia
- weight loss
- feeling of discomfort
- urinary tract infection
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Epidyolex
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the bottle. The expiration date is the last day of the month indicated.
If you have solution left in the bottle more than 12 weeks after opening it for the first time, do not use it.
This medicine does not require special storage conditions.
Medicines should not be thrown away through wastewater or household waste. Ask your pharmacist how to dispose of the medicine you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Epidyolex
- The active ingredient is cannabidiol. Each ml of oral solution contains 100 mg of cannabidiol.
- The other components are refined sesame oil, anhydrous ethanol, sucralose, and strawberry flavor (including benzyl alcohol) (see section 2).
Appearance of Epidyolex and Package Contents
Epidyolex is a clear and colorless to yellowish oral solution. It is supplied in an amber glass bottle with a child-resistant screw cap.
The following package sizes are available for Epidyolex:
100 ml (1 bottle of 100 ml) with 2 oral dosing syringes calibrated to 5 ml, 2 to 1 ml, and two adapters for the bottle.
300 ml (3 bottles of 100 ml) with 2 oral dosing syringes calibrated to 5 ml, 2 to 1 ml, and three adapters for the bottle.
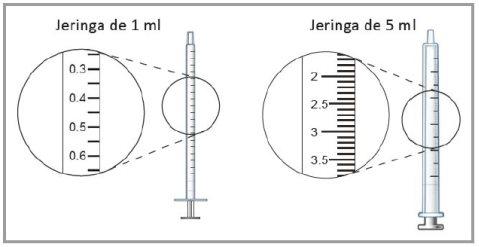
The 5 ml syringes are graduated in increments of 0.1 ml, and the 1 ml syringes have increments of 0.05 ml.
Only some package sizes may be marketed.
Marketing Authorization Holder
Jazz Pharmaceuticals Ireland Ltd
5th Floor
Waterloo Exchange
Waterloo Road
Dublin 4
D04 E5W7
Ireland
Email: [email protected]
Manufacturer
Jazz Pharmaceuticals Netherlands B.V.,
Smallepad 32, 3811MG, Amersfoort,
Netherlands
Email: [email protected]
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Tel: +31 207176898 | Lietuva Tel: +353 1 968 1631 |
| Luxembourg/Luxemburg Tel: +31 207176898 |
Ceská republika Tel: +353 1 968 1631 | Magyarország Tel: +353 1 968 1631 |
Danmark Tlf: +45 69918419 | Malta Tel: +353 1 968 1631 |
Deutschland Tel: +49(0)3022957821 | Nederland Tel: +31 207176898 |
Eesti Tel: +353 1 968 1631 | Norge Tlf: +353 1 968 1631 |
Ελλάδα Τηλ: +353 1 968 1631 | Österreich Tel: +353 1 968 1631 |
España Jazz Pharmaceuticals Iberia, S.L. Tel: +34 914142493 | Polska Tel: +353 1 968 1631 |
France Exploitant: Jazz Pharmaceuticals France SAS Tél: +33 176728925 | Portugal Tel: +351 308805626 |
Hrvatska Tel: +353 1 968 1631 | România Tel: +353 1 968 1631 |
Ireland Tel: +353 1 968 1631 | Slovenija Tel: +353 1 968 1631 |
Ísland Sími: +353 1 968 1631 | Slovenská republika Tel: +353 1 968 1631 |
Italia Jazz Healthcare Italy S.r.l. Tel: +39 (0)800959164 | Suomi/Finland Puh/Tel: +353 1 968 1631 |
Κύπρος Τηλ: +353 1 968 1631 | Sverige Tel: +46 406688521 |
Latvija Tel: +353 1 968 1631 |
Date of the Last Revision of this Prospectus: 08/2024
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites about rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EPIDYOLEX 100 mg/ml ORAL SOLUTIONDosage form: TABLET, 100 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: TABLET, 200 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: TABLET, 25 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription required
Online doctors for EPIDYOLEX 100 mg/ml ORAL SOLUTION
Discuss questions about EPIDYOLEX 100 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions